Chapter 14 Part 3 - Mass Spectrometry (MS)
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
M+ Peak
Shows molecular weight of the compound.
Nitrogen Rule
Odd # of N = Odd # molecular weight.
Contains I
Peak at 127 m/z.
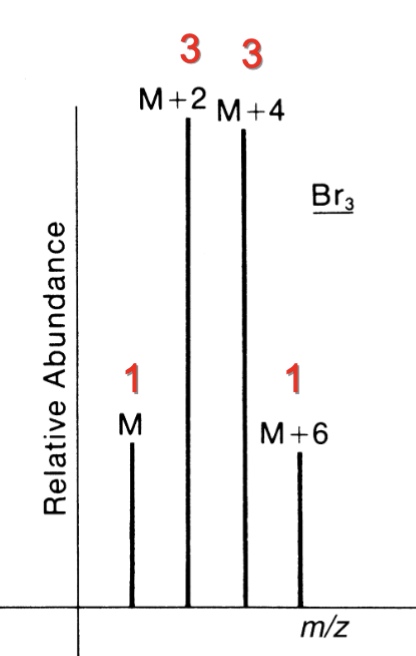
Contains 1 Br
M : M+2
1:1
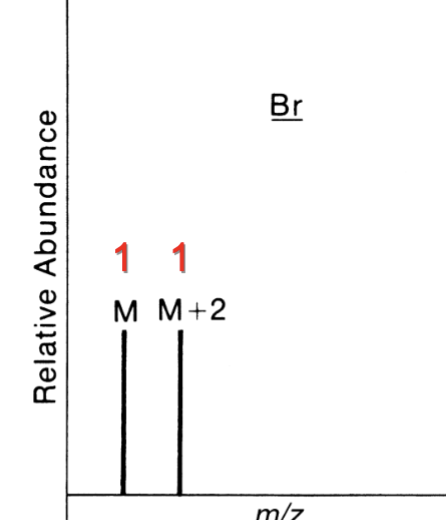
Contains 2 Br
M : M+2 : M+4
1 : 2 : 1
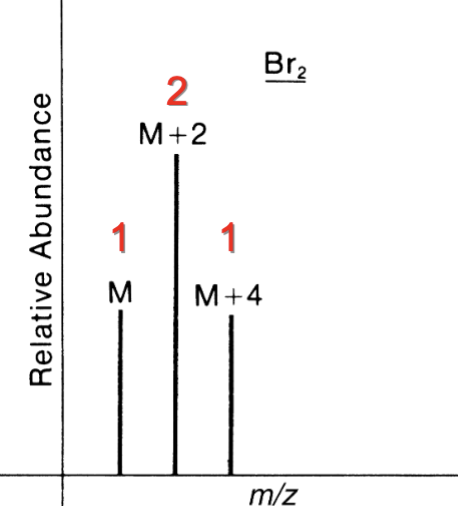
Contains 3 Br
M : M+2 : M+4 : M+6
1 : 3 : 3 :1
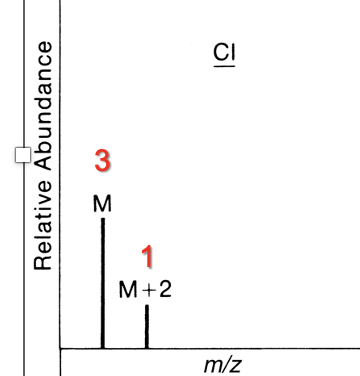
Contains 1 Cl
M : M+2
3 : 1
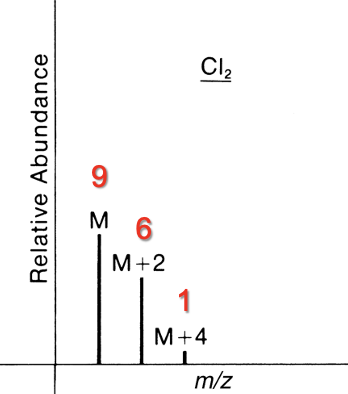
Contains 2 Cl
M : M+2 : M+4
9 : 6 : 1
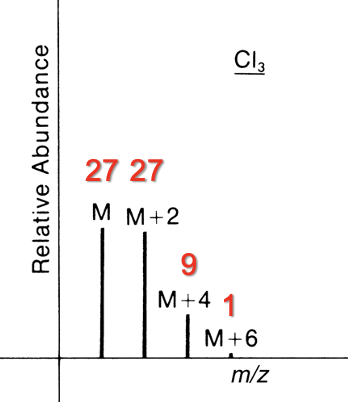
Contains 3 Cl
M : M+2 : M+4 : M+6
27 : 27 : 9 : 1
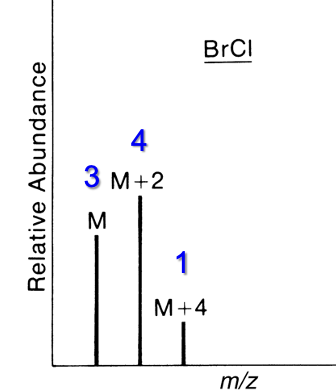
Contains 1 Br and 1 Cl
M : M+2 : M+4
3 : 4 : 1
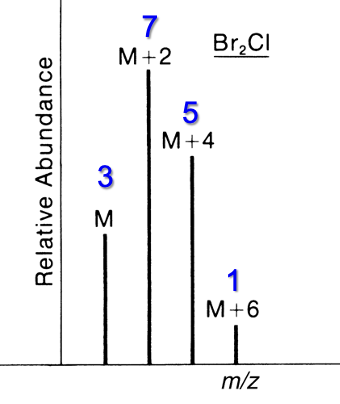
Contains 2 Br and 1 Cl
M : M+2 : M+4 : M+6
3 : 7 : 5 : 1
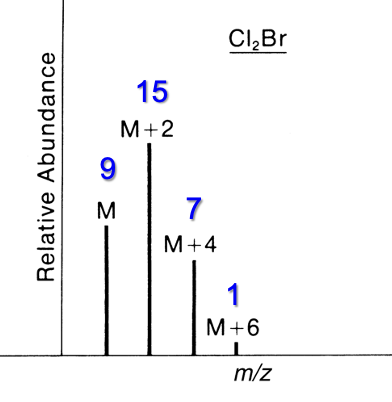
Contains 1 Br and 2 Cl
M : M+2 : M+4 : M+6
9 : 15 : 7 : 1
Rule of 13
[M+] / 13 = n + (r/13)
Based Formula = CnHn+r
![<p>[M<sup>+</sup>] / 13 = n + (r/13)</p><p>Based Formula = C<sub>n</sub>H<sub>n+r</sub></p>](https://knowt-user-attachments.s3.amazonaws.com/31085580-b873-4237-aeae-fa6b8b7ef9cd.png)
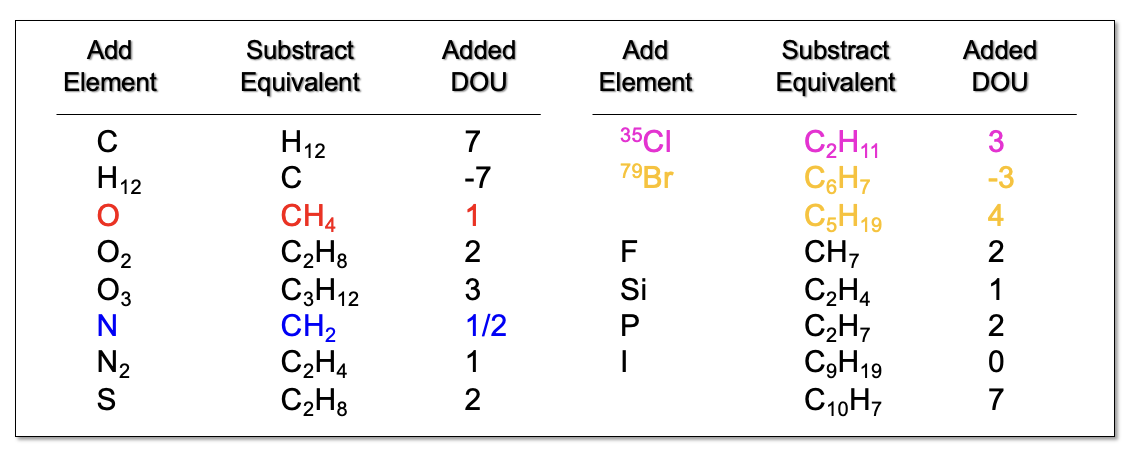
Common Elements (MW and Subtract Equivalent)
C
O
N
Cl
Br
Common Elements (MW and Subtract Equivalent)
C - 12 - H12
O - 16 - CH4
N - 14 - CH2
Cl - 35 - C2H11
Br - 79 - C6H7
Why do M+1 / M+2 etc… peaks form?
Due to C / Cl / Br / etc… isotopes.