532 Lec 26-27
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
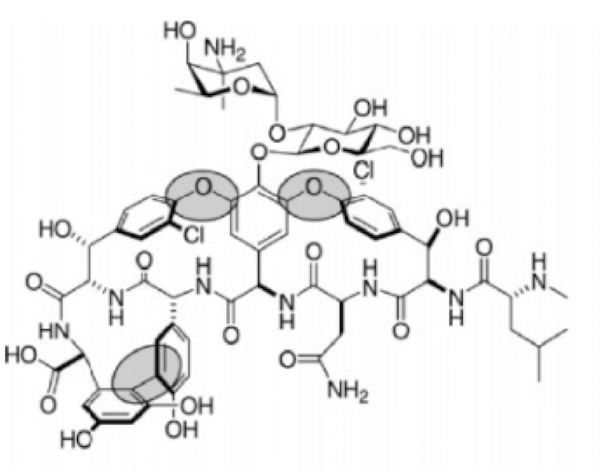
what drug
vancomycin
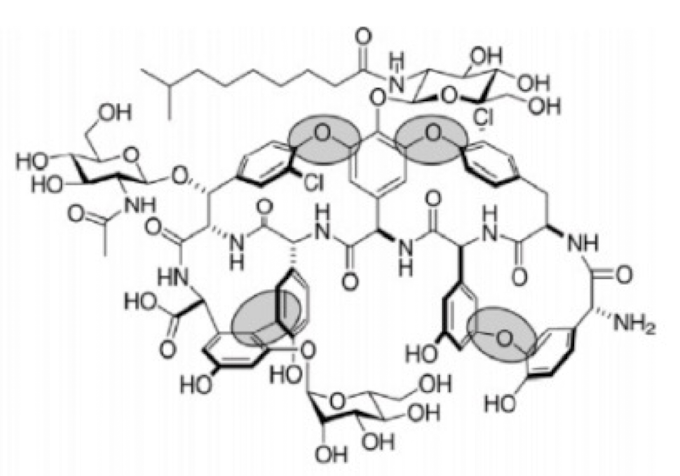
what drug
teicoplanin
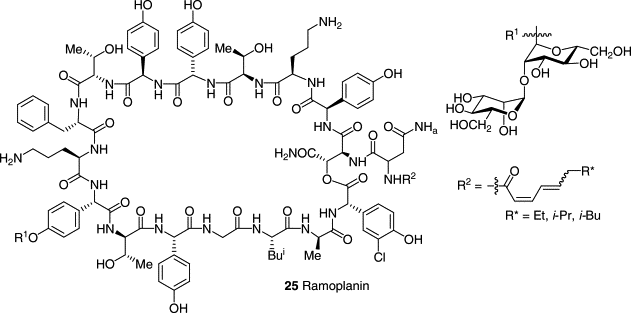
ramoplanin looks like this
Talavancin (Vibativ), Dalbavancin, Chlorobiphenyl Vancomycin, Oritavancin,
Target bacteria cell wall synthesis
Transpeptidation inhibitors
bind directly to the D-alanyl-D-alanine termini of the peptidoglycan precursors.
Why are vancomycin, teicoplanin and ramoplanin effective only against gram positive
Microbes?
Vancomycin, teicoplanin, and ramoplanin are glycopeptide antibiotics effective against Gram-positive bacteria because they target the synthesis of the bacterial cell wall, specifically interfering with peptidoglycan formation
In contrast, Gram-negative bacteria have a different cell wall structure, preventing glycopeptide antibiotics from reaching their target.
2. Know how vancomycin (and by inference teicoplanin) inhibits both transpeptidases
and transglycosidases in numerous bacteria. Be able to show how different parts of
vancomycin interact with the peptide sequence D-ala, D-ala, L-lys and also what parts of
the molecule appear to mimic the NAG-NAM glycan part of the peptidoglycan
monomers when binding to transglycosidases.
it blocks it
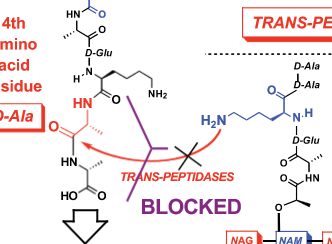
Understand why the switching over of bacteria from using D-ala, D-ala, L-lys to D-lac,
D-ala, L-lys leads to a loss in activity of vancomycin. Bear in mind the importance of lost
H-bond but also introduced repulsive interactions between electron lone pairs on
vancomycin and the “new” D-lac, D-ala, L-lys binding partner. Know that in the case of
vancomycin how this translates to about a 1000-fold loss in binding affinity of
vancomycin for its target peptide sequence. Be able to provide a solution to the problem
just as we talked about in class with the replacement of a vancomycin amide with a
secondary amine.
ugh
Why are talavancin, dalbavancin, chlorobiphenyl vancomycin
& oritavancin better able to inhibit transglycosidase relative to
Vancomycin?
because they seem to bind better seems to correlate
to increased lipophilicity in the sugar binding components of vanco analogs.
structural differences between Talavancin (Vibativ – approved 2009) and vancomycin that lend talavancin a greater degree of antibacterial activity.
Talavancin, unlike vancomycin, has a lipophilic tail that helps it penetrate bacterial membranes more effectively. This, combined with its dual mode of action of inhibiting cell wall synthesis and disrupting membrane integrity, makes talavancin more potent against a wider range of Gram-positive bacteria
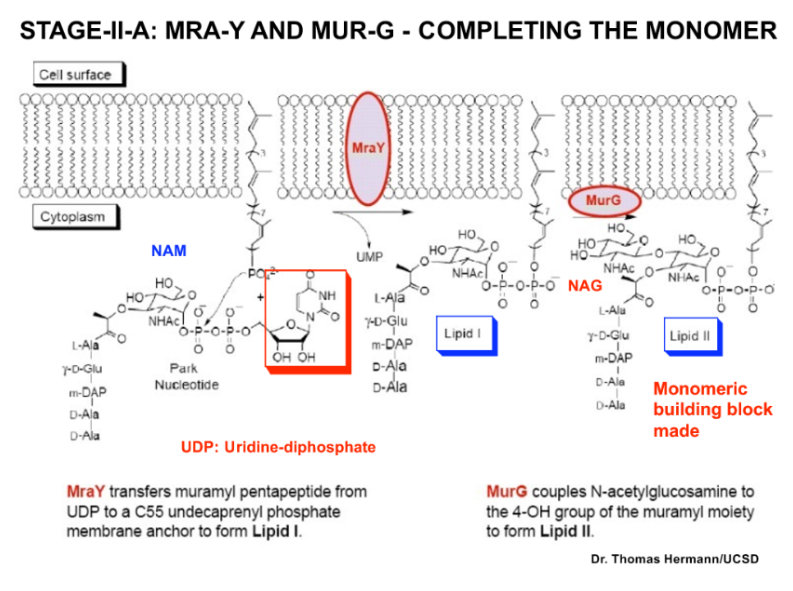
MraY and MurG enzymes roles in peptidoglycan monomer production
MraY and MurG are enzymes involved in peptidoglycan synthesis. MraY transfers the peptidoglycan precursor Lipid I to the bacterial membrane, while MurG adds N-acetylglucosamine to form Lipid II.
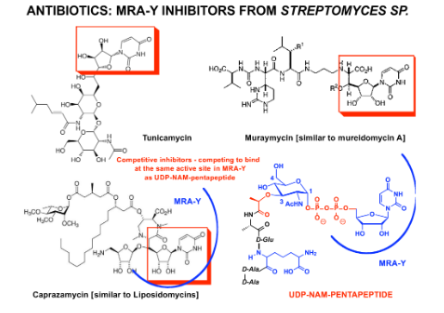
how MraY is inhibited by tunicamycin, muraymycin, and caprazamycin. What part of
these structures is isosteric with the key starting material to “Lipid I”?
Tunicamycin, muraymycin, and caprazamycin inhibit MraY by mimicking the nucleoside part of Lipid I, blocking its synthesis.
The nucleoside moiety in tunicamycin, muraymycin, and caprazamycin is isosteric with the key starting material for "Lipid I."
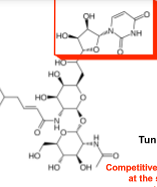
what drug
tunicamycin
what ramoplanin is, how it is similar and how it is dissimilar to vancomycin.
How does ramoplanin appear to demonstrate antibacterial activity?
→Possesses the same mechanism of action as Vancomycin
→Binds the entire Lipid-II/NAM-NAG unit:
Blocks trans-glycosylation and likely also trans-peptidation. Similar to Vanco in that it appears to be a dual inhibitor
→Ramoplanin is approved by FDA, but is unstable in the blood, can only be used orally
What drug and MOA
erythromycin (ketolides) MOA
etolide - bind 50S and blocks peptide exit tunnel → inhibits bacterial protein synthesis
tygacil
glycyclines - block entry of aminoacyl-tRNA into ribosome’s A site → inhibits bacterial protein synthesis
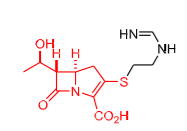
Whar drug and MOA
Imipenem
new carbapenems
imipenem
meropenem
doripenem
razupenem
carbapenems MOA
Beta-lactam MOA
fluoroquinolones
ciprofloxacin
levofloxacin
finafloxacin
JNJ-Q2 (still in trials)
fluoroquinolone MOA
bacterial DNA gyrase inhibitor → inhibits DAN replication
Fidaxomicin MOA
Inhibits bacterial RNA polymerase
Bedaquiline MOA
Bacterial ATP synthase proton pump inhibitor