Electron & Molecular Geometry
1/34
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
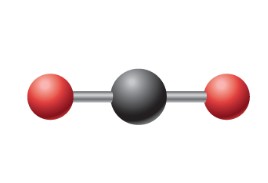
What is this electron geometry?
Linear: 2 electron groups around central atom
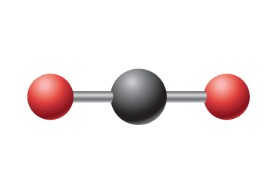
What is the ideal angle for this electron geometry?
Linear: 180 degrees
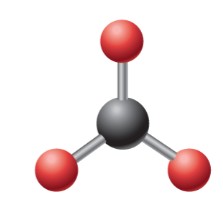
What is the electron geometry?
Trigonal Planar: 3 electron groups around central atom
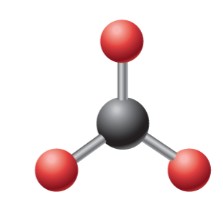
What is the ideal angle for this electron geometry?
Trigonal Planar: 120 degrees
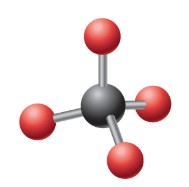
What is the electron geometry?
Tetrahedral: 4 electron groups around central atom
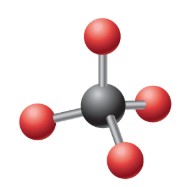
What is the ideal angle for this electron geometry?
Tetrahedral: 109.5 degrees
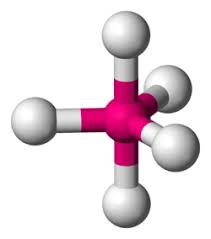
What is the electron geometry?
Trigonal Bipyramidal: 5 electron groups around central atom
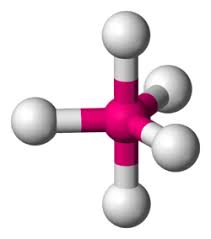
What are the ideal angles for this electron geometry?
Trigonal Bipyramidal: 90, 120, and 180 degrees
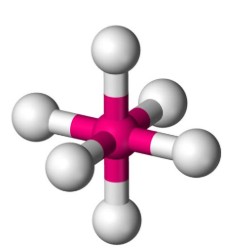
What is the electron geometry?
Octahedral: 6 electron groups around central atom

What are the ideal angles for this electron geometry?
Octahedral: 90 and 180 degrees
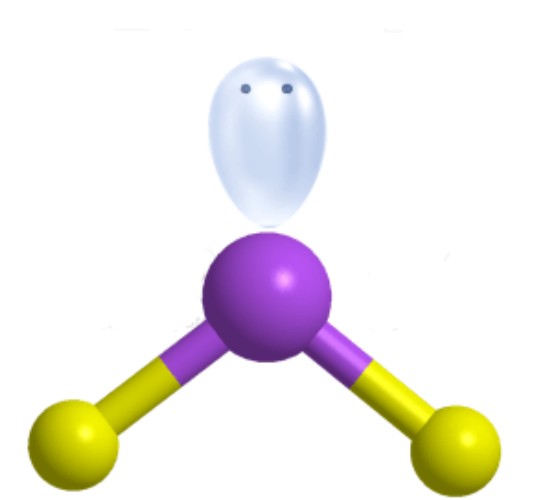
What is the molecular geometry?
Bent [Trigonal Planar]: 1 lone pair apart of three electron groups around central atom
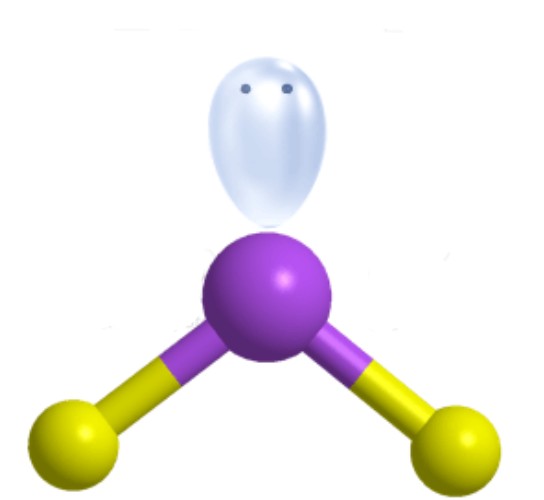
What is the ideal angle for this molecular geometry?
Bent [Trigonal Planar]: 120 degrees
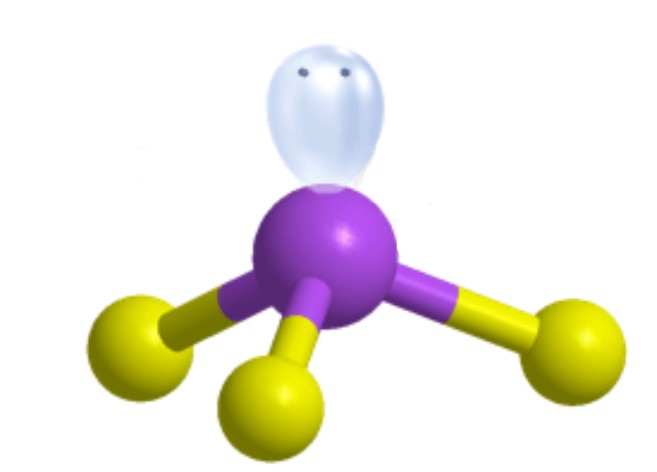
What is the molecular geometry?
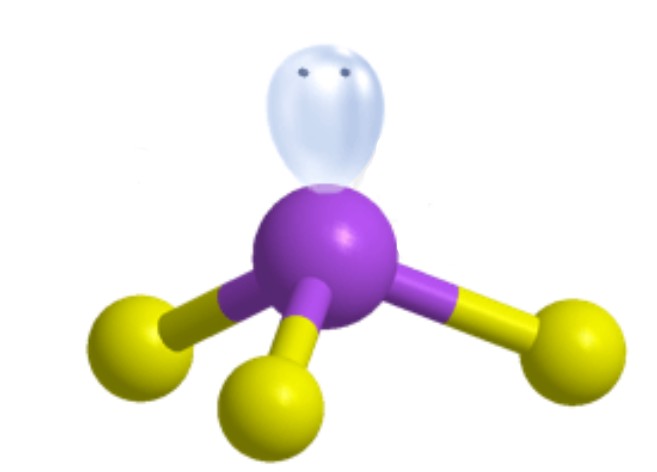
Trigonal Pyramidal [Tetrahedral]: 1 lone pair apart of four electron groups around central atom
What is the ideal angle for this molecular geometry?
Trigonal Pyramidal [Tetrahedral]: 109.5 degrees
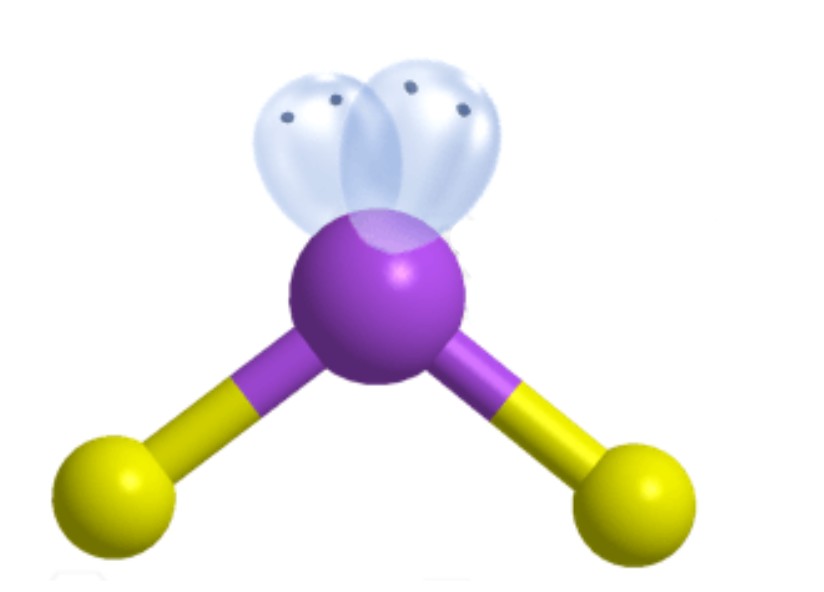
What is the molecular geometry?
Bent [Tetrahedral]: 2 lone pairs apart of four electron groups around central atom
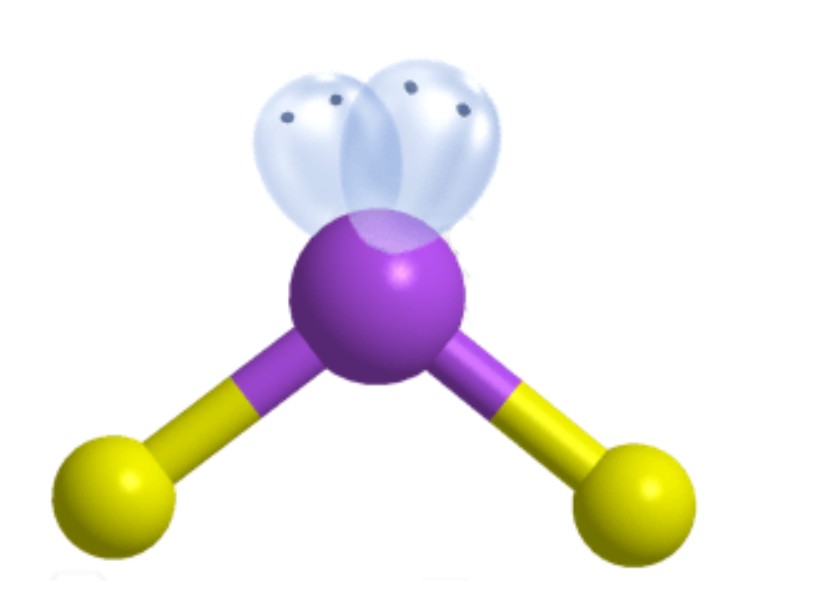
What is the ideal angle for this molecular geometry?
Bent [Tetrahedral]: 109.5 degrees
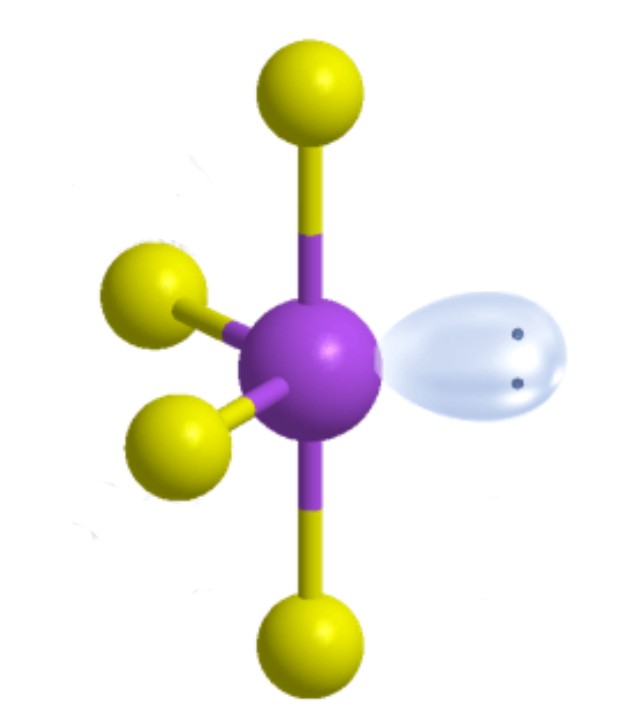
What is the molecular geometry?
Seesaw [Trigonal Bipyramidal]: 1 lone pair apart of five electron groups around central atom

What are the ideal angles for this molecular geometry?
Seesaw [Trigonal Bipyramidal]: 90, 120, and 180 degrees
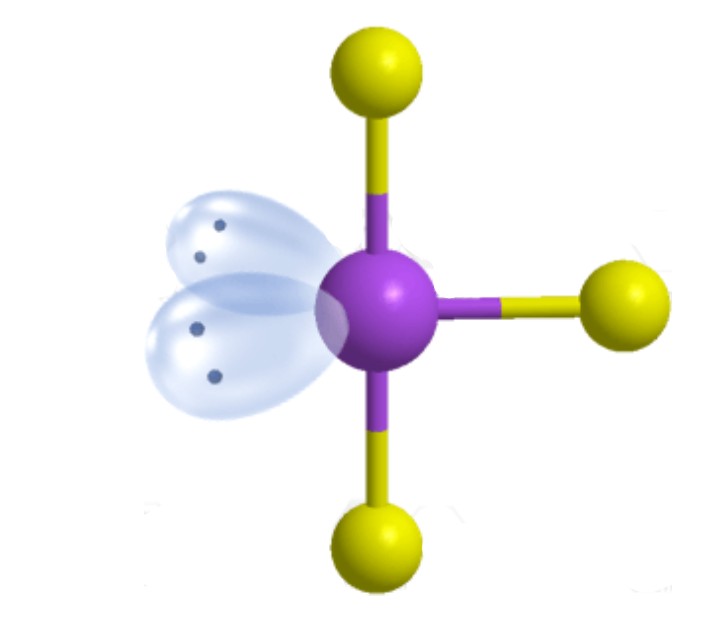
What is the molecular geometry?
T-Shaped [Trigonal Bipyramidal]: 2 lone pairs apart of five electron groups around central atom
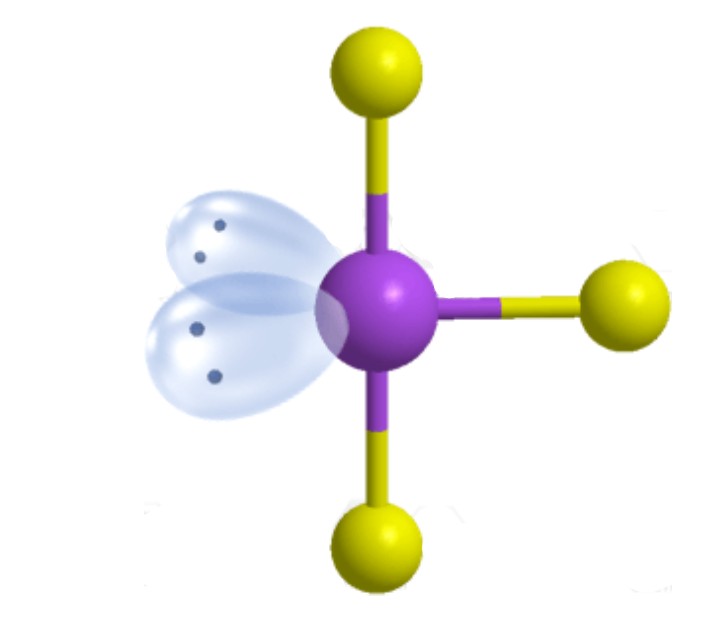
What are the ideal angles for this molecular geometry?
T-Shaped [Trigonal Bipyramidal]: 90 and 180 degrees
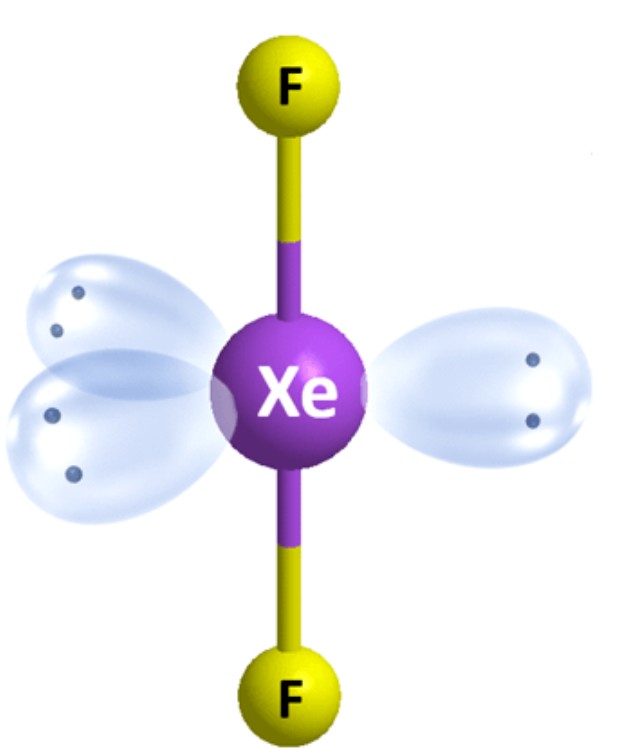
What is the molecular geometry?
Linear [Trigonal Bipyramidal]: 3 lone pairs apart of five electron groups around central atom
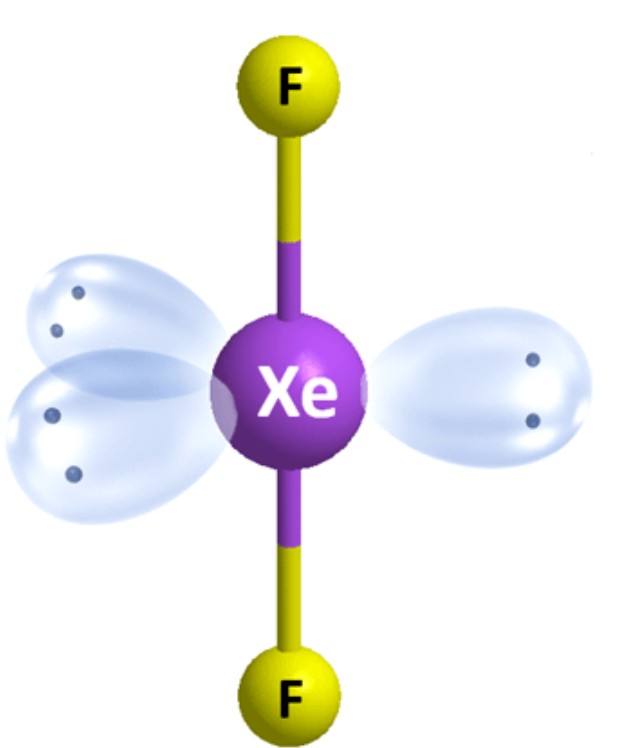
What is the ideal angle for this molecular geometry?
Linear [Trigonal Bipyramidal]: 180 degrees
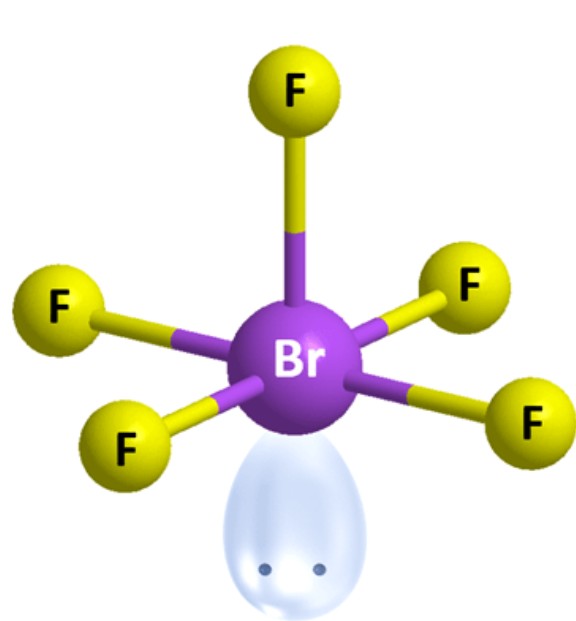
What is the molecular geometry?
Square Pyramidal [Octahedral]: 1 lone pair apart of six electron groups around central atom
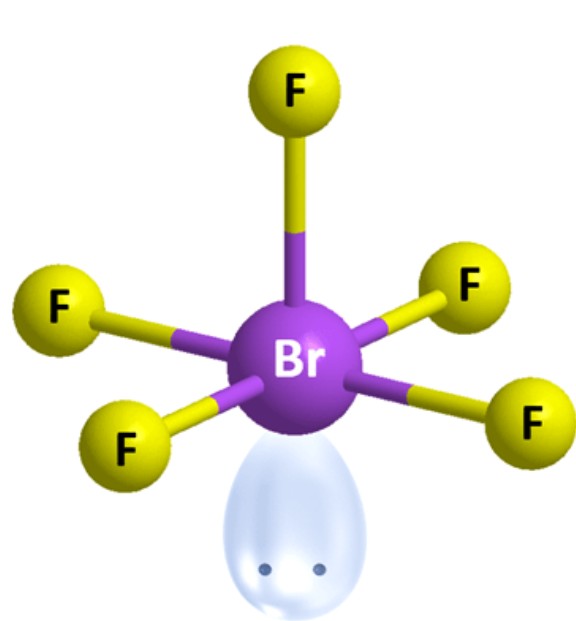
What are the ideal angles for this molecular geometry?
Square Pyramidal [Octahedral]: 90 and 180 degrees
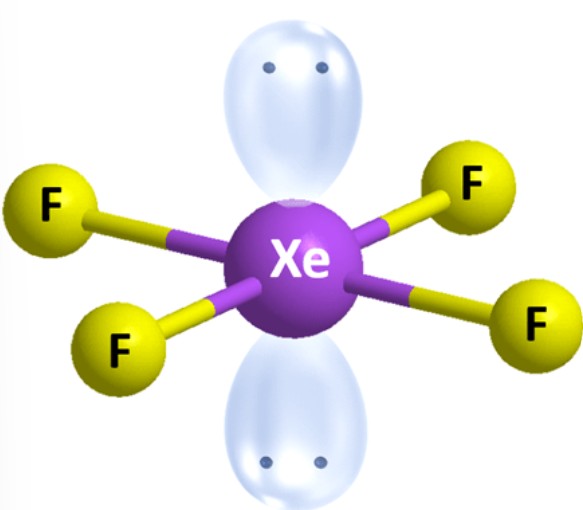
What is the molecular geometry?
Square Planar [Octahedral]: 2 lone pairs apart of six electron groups around central atom
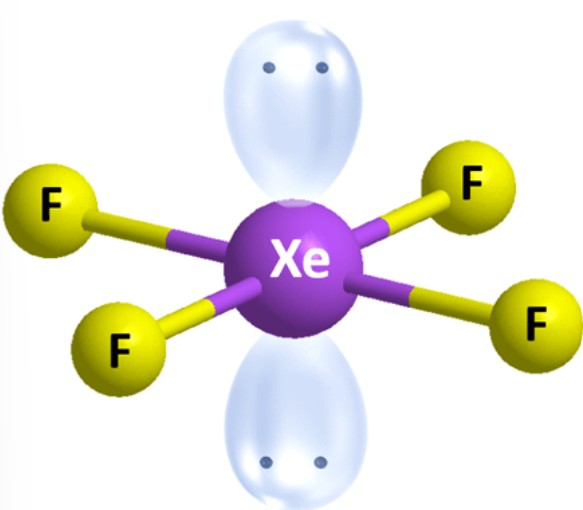
What are the ideal angles for this molecular geometry?
Square Planar [Octahedral]: 90 and 180 degrees
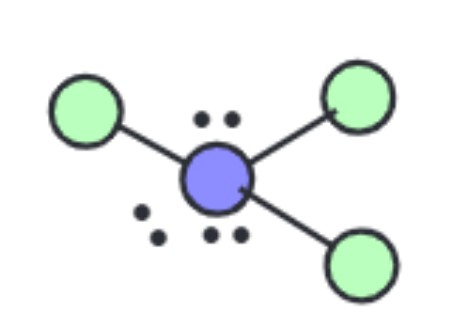
What is the molecular geometry?
T-Shaped [Octahedral]: 3 lone pairs apart of six electron groups around central atom
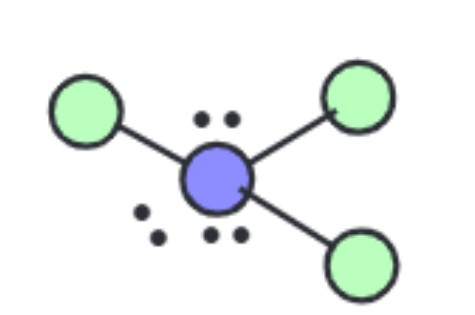
What are the ideal angles for this molecular geometry?
T-Shaped [Octahedral]: 90 and 180 degrees
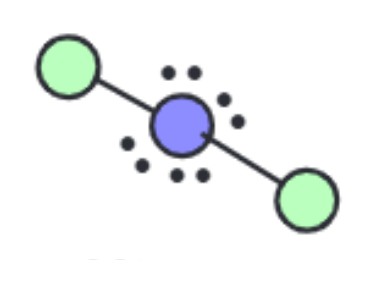
What is the molecular geometry?
Linear [Octahedral]: 4 lone pairs apart of six electron groups around central atom
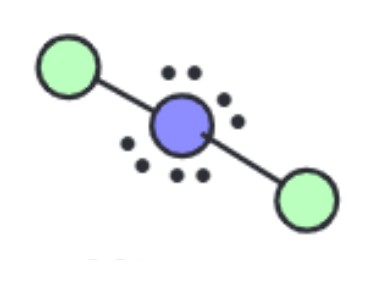
What is the ideal angle for this molecular geometry?
Linear [Octahedral]: 180 degrees

What is the hybridization of a Linear EG?
sp
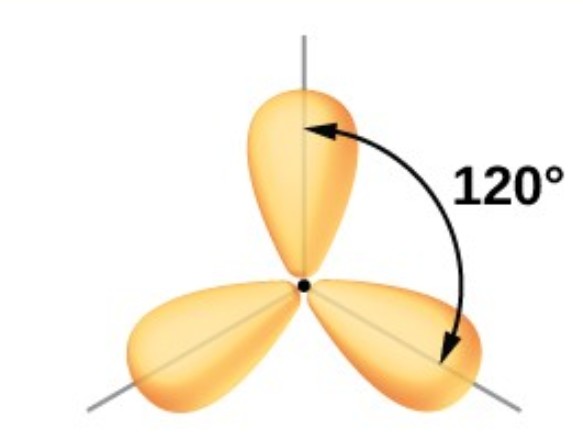
What is the hybridization of a Trigonal Planar EG?
sp²
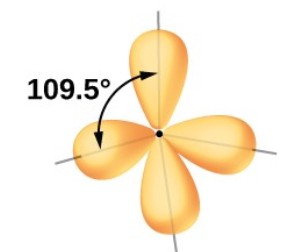
What is the hybridization of a Tetrahedral EG?
sp³
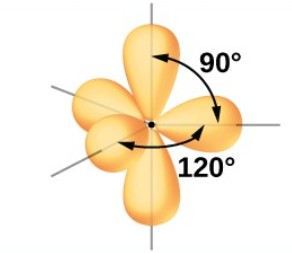
What is the hybridization of a Trigonal Bipyramidal EG?
sp³d
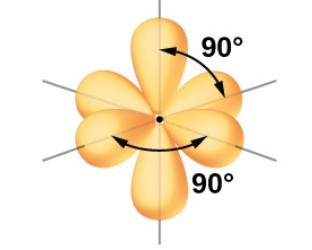
What is the hybridization of an Octahedral EG?
sp³d²