BS 181H Exam 1
5.0(1)
Card Sorting
1/189
Earn XP
Description and Tags
Last updated 5:11 AM on 2/1/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
190 Terms
1
New cards
2
New cards
Biology
the study of living organisms and their environments at various levels of organization
3
New cards
what are the levels of biological organization (in order)
atoms, molecules, macromolecules, cells, tissues, organs, organism, population, community, ecosystem, biosphere
4
New cards
organism
living things of a species
(levels of biological organization)
(levels of biological organization)
5
New cards
population
organisms of the same species that occupy the same environment
(levels of biological organization)
(levels of biological organization)
6
New cards
community
populations of different species
(levels of biological organization)
(levels of biological organization)
7
New cards
ecosystem
formed by the interactions of a community of organisms with their environment
(levels of biological organization)
(levels of biological organization)
8
New cards
biosphere
all the places on earth where organisms exist
(levels of biological organization)
(levels of biological organization)
9
New cards
evolution
diversity of life evolved through mutation, natural selection, and genetic exchange
(core concepts of biology)
(core concepts of biology)
10
New cards
structure and function
basic units of structure defining the function of all living things
(core concepts of biology)
(core concepts of biology)
11
New cards
information flow, exchange, and storage
traits and behavior of organisms that happen to some extent from the control by the expression of genetic information
(core concepts of biology)
(core concepts of biology)
12
New cards
pathways and transformations of energy and matter
biological processes based on pathways transforming chemicals and governed by laws of thermodynamics
(core concepts of biology)
(core concepts of biology)
13
New cards
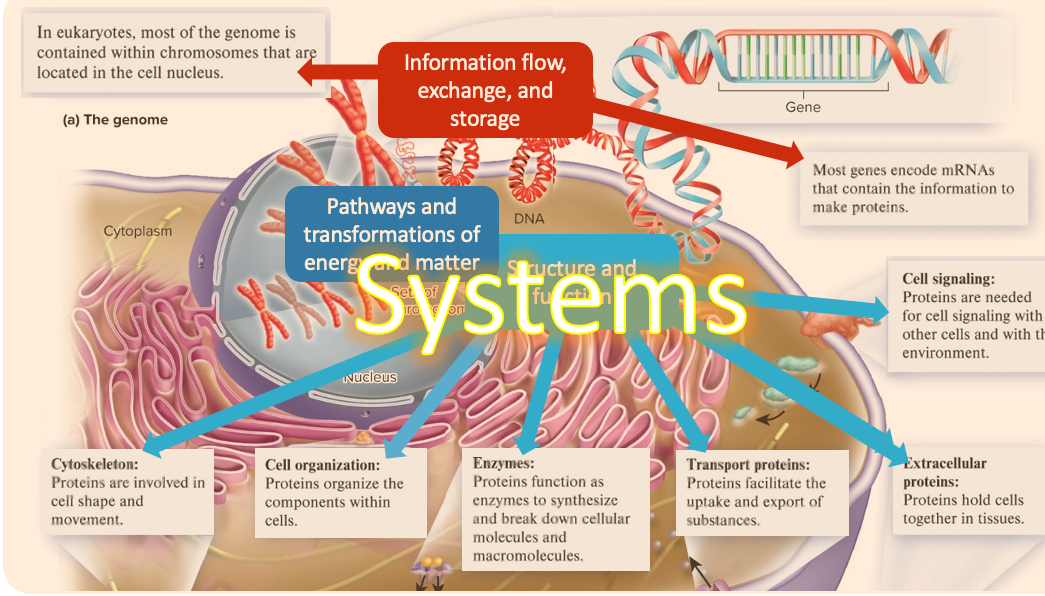
systems
living systems interconnected and interacting
(core concepts of biology)
(core concepts of biology)
14
New cards
Unity (mechanism of evolution)
* all life displays a common set of characteristics (ex: homeostasis, cellular organization, ability to reproduce)
* united by a shared evolutionary history
* united by a shared evolutionary history
15
New cards
evolution (mechanism of evolution)
* heritable change in **genetic material** in a population of organisms from one generation to the next
* the genetic material mostly composed of DNA
* DNA provides a blueprint for traits and function
* DNA is heritable
\
* lead to diversity to form in diverse environments
* the genetic material mostly composed of DNA
* DNA provides a blueprint for traits and function
* DNA is heritable
\
* lead to diversity to form in diverse environments
16
New cards
genome
* the complete genetic material of an organism
* segments of DNA
* govern the traits of organisms
* most genes are transcribed into mRNAs
* most mRNAs are translated into a polypeptide
* a protein may compose more than 1 polypeptides
* segments of DNA
* govern the traits of organisms
* most genes are transcribed into mRNAs
* most mRNAs are translated into a polypeptide
* a protein may compose more than 1 polypeptides
17
New cards
genomics
**the study of genomes** and many if not all genes at the same time
18
New cards

characteristics and importance of a model system
a species that is straightforward to study where knowledge gained can potentially be useful for understanding other species
19
New cards
hypothesis
* a proposed explanation for a phenomenon
* based on prior knowledge
* must be testable (can be shown to be correct or incorrect)
* support/reject with evidence
* __**never**__ proven with certainty
ex: Maple trees drop their leaves in autumn because of shorten hours of sunlight.
* based on prior knowledge
* must be testable (can be shown to be correct or incorrect)
* support/reject with evidence
* __**never**__ proven with certainty
ex: Maple trees drop their leaves in autumn because of shorten hours of sunlight.
20
New cards
theory
* a broad explanation of aspects of the natural substantiated by a large body of evidence
* allows us to make many predictions
* can __**never**__ be proven true
* due to overwhelming evidence, __**very likely**__ to be true
ex: DNA is the genetic material
* allows us to make many predictions
* can __**never**__ be proven true
* due to overwhelming evidence, __**very likely**__ to be true
ex: DNA is the genetic material
21
New cards
discovery based hypothesis
* inductive reasoning
* data → patterns → conclusions
\
ex: test candidate drugs to look for action against disease
ex: sequence genomes and proteomes
* data → patterns → conclusions
\
ex: test candidate drugs to look for action against disease
ex: sequence genomes and proteomes
22
New cards
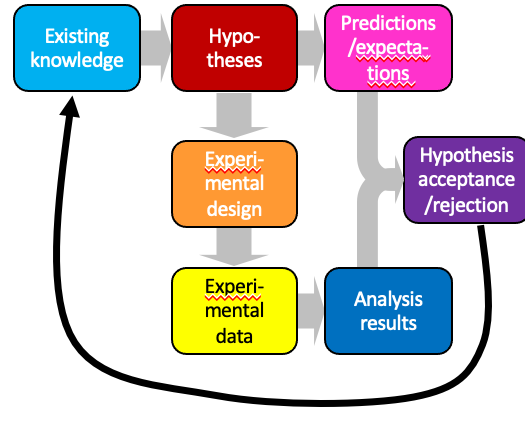
hypothesis-based science
* deductive reasoning
23
New cards
purpose of controls in experiments
increase the reliability of results and also to eliminate errors and bias
24
New cards
control group
the group that does not receive the new treatment being studied
25
New cards
experimental group
the group that does receive the new treatment being studied
26
New cards
synthesizing life-like systems (grand challenges in biology)
Can we construct systems with characteristics of life that are capable synthesizing materials or carrying out functions as yet unseen in natural biology? (This is why it’s challenging)
27
New cards
understanding the brain (grand challenges in biology)
the human brain may be nature’s most complex system. Just how do all the neurons and synapse work together and contribute to brain functions?
28
New cards
predicting characteristic based on DNA (grand challenges in biology)
Ultimately, the blueprint for form and function lies in an organism’s DNA sequence. How can DNA be used to predict forms and functions? (This is why it’s challenging)
29
New cards
interactions of the earth, its climate, and the biosphere (grand challenges in biology)
how do these processes operate on much different scales of time (fractions of a second to many years) and space (microscopic to the global) unfold? (This is why it’s challenging)
30
New cards
atom
the smallest functional units of matter that form all chemical substances
* cannot be further broken down into other substances by ordinary means
* 2 or more bonds bonded together to form molecules
* each specific type of atom is a chemical element
* cannot be further broken down into other substances by ordinary means
* 2 or more bonds bonded together to form molecules
* each specific type of atom is a chemical element
31
New cards
structure of an atom
dense nucleus that contains protons and neutrons surrounded by a “cloud” of electrons
* # of protons = # of electrons (unless the atom is charged)
* # of protons = atomic number
* # of protons = # of electrons (unless the atom is charged)
* # of protons = atomic number
32
New cards
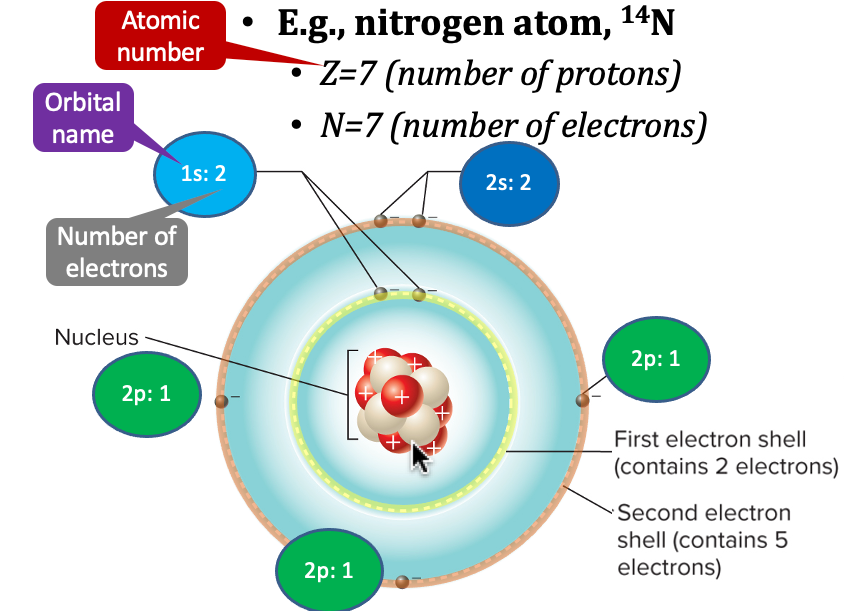
orbitals
regions surrounding the nucleus in which the probability of finding electrons is high
* a central nucleus surrounded by cloudlike orbitals
\
picture:
* 7 protons and 7 electrons
* 2 protons fill the 1st shell
* 2 in the 1s orbital
* 5 electrons in the 2nd shell
* 2 fill the 2s orbital
* 1 in each of the three 2p orbitals
* electrons in the outer shell available to combine with other atoms are called valence electrons
* a central nucleus surrounded by cloudlike orbitals
\
picture:
* 7 protons and 7 electrons
* 2 protons fill the 1st shell
* 2 in the 1s orbital
* 5 electrons in the 2nd shell
* 2 fill the 2s orbital
* 1 in each of the three 2p orbitals
* electrons in the outer shell available to combine with other atoms are called valence electrons
33
New cards
about **12C, which of the following is correct?**
\
a) it has 12 protons
b) it has 12 electrons
c) it’s 2p orbitals contains 4 electrons
d) it has 4 valence electrons
\
a) it has 12 protons
b) it has 12 electrons
c) it’s 2p orbitals contains 4 electrons
d) it has 4 valence electrons
D
\
a) it has 12 protons (actually has 6)
b) it has 12 electrons (actually has 6)
c) it’s 2p orbitals contain 4 electrons (actually has 2 electrons bc the electron configuration is 1s1 1s2 2s1 2s2 2p1 **2p2**)
d) it has 4 valence electrons (6-2 = 4… 6 is from carbon’s atomic number and 2 is from helium’s noble number)
\
a) it has 12 protons (actually has 6)
b) it has 12 electrons (actually has 6)
c) it’s 2p orbitals contain 4 electrons (actually has 2 electrons bc the electron configuration is 1s1 1s2 2s1 2s2 2p1 **2p2**)
d) it has 4 valence electrons (6-2 = 4… 6 is from carbon’s atomic number and 2 is from helium’s noble number)
34
New cards
results of Rutherford’s experiment
Rutherford conducted the gold foil experiment where an alpha particle is shot to a gold foil surrounded by a detection screen. Most of the alpha particles went straight through the gold foil (undeflected), some slightly deflected, and very few alpha particles bounced back. Through this experiment, it was proven that atoms are mostly composed of mostly empty space with positive charges in a small volume (the nucleus)
35
New cards
elements that make up most of living organisms
oxygen, carbon, hydrogen, and nitrogen
\
* hydrogen and oxygen occur primarily in water
* nitrogen is found in proteins
* carbon is the building block of all living matter
\
* hydrogen and oxygen occur primarily in water
* nitrogen is found in proteins
* carbon is the building block of all living matter
36
New cards
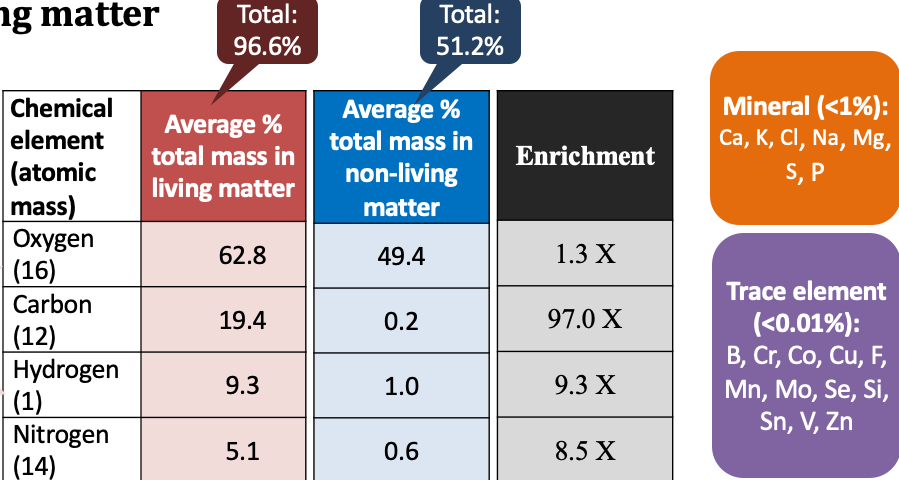
most oxygen and hydrogen atoms in living things are from water (2H and 1O). **Why does the enrichment numbers differ so much from 2-to-1 ratio?**
because 1) the atomic masses differ by 16x between O and H, 2) in addition to water, H is predominantly associated witH C in macromolecules
37
New cards
molecule
more than 2 atoms bonded together
38
New cards
compound
a molecule composed of 2 or more elements
39
New cards
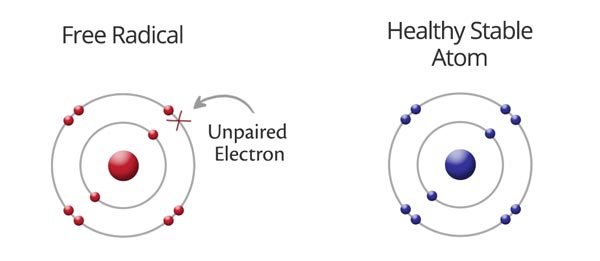
free radicals
molecules containing an atom with a **single, unpaired electron** in its outer shell (oxidized)
* can form by exposure to radiation and some toxins
* highly reactive
* **can “steal” an electron from other molecules**
* can cause cell damage
* can kill invading bacteria
* benefits of antioxidants
* can form by exposure to radiation and some toxins
* highly reactive
* **can “steal” an electron from other molecules**
* can cause cell damage
* can kill invading bacteria
* benefits of antioxidants
40
New cards
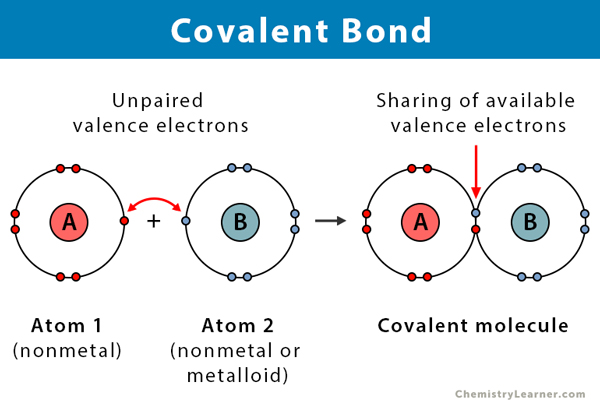
covalent bonds
electrons are shared to fill valence shells, and can be polar or nonpolar
* atoms share electron pair(s)
* between atoms with unfilled outer electron shells
* strong chemical bond
* can share:
* 1 electron pair: single bond
* 2 pairs double bond
* 3 pairs: triple bond
* atoms are stable when their **outer shell** is full (8 valence electrons)
* for many atoms, the outer shell is filled with **8 electrons** (“the octet rule”)
* any exception is HYDROGEN, which fills its outer shell with just 2 electrons
* atoms share electron pair(s)
* between atoms with unfilled outer electron shells
* strong chemical bond
* can share:
* 1 electron pair: single bond
* 2 pairs double bond
* 3 pairs: triple bond
* atoms are stable when their **outer shell** is full (8 valence electrons)
* for many atoms, the outer shell is filled with **8 electrons** (“the octet rule”)
* any exception is HYDROGEN, which fills its outer shell with just 2 electrons
41
New cards
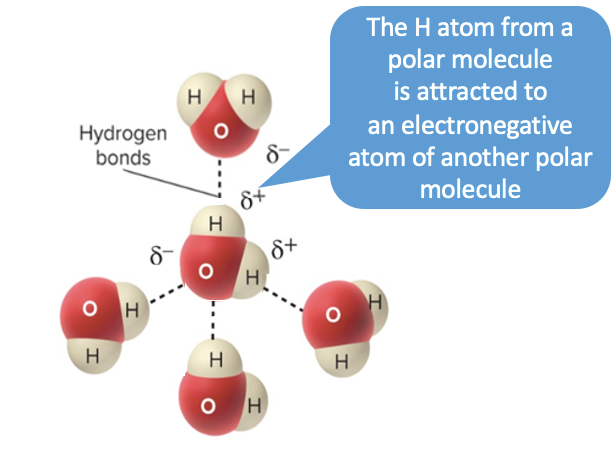
hydrogen bonds
hydrogen atom from **one polar molecule** attracted to an **electronegative atom** from another molecule
* small molecules may bind to enzymes through hydrogen bonds
* ex: bonds between DNA strands, small molecules and enzymes
* small molecules may bind to enzymes through hydrogen bonds
* ex: bonds between DNA strands, small molecules and enzymes
42
New cards
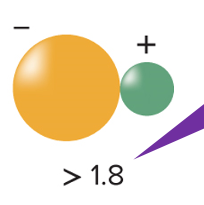
ionic bonds
electrons are transferred, forming ions that are attached to each other
* forms when differences in electronegativities are large between 2 atoms
* forms when differences in electronegativities are large between 2 atoms
43
New cards

Nonpolar covalent bond
similar electronegativities between atoms connected by the bond (equal sharing of electrons)
* δ: attraction to electrons
* no charge difference across molecules
* ex: C-C, C-H (they have very similar electronegativities)
* δ: attraction to electrons
* no charge difference across molecules
* ex: C-C, C-H (they have very similar electronegativities)
44
New cards
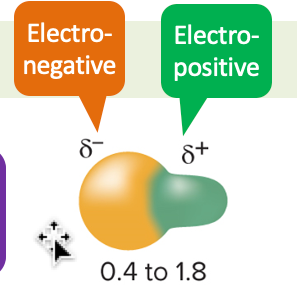
polar covalent bond
different electronegativities between atoms connected by the bond (unequal sharing of electrons)
* electronegative side (more electrons) and electropositive side (less electrons)
* since one atom is more electronegative, it will attract more of the electrons, causing the unequal sharing of electrons
* ex: O-H, N-H
* electronegative side (more electrons) and electropositive side (less electrons)
* since one atom is more electronegative, it will attract more of the electrons, causing the unequal sharing of electrons
* ex: O-H, N-H
45
New cards
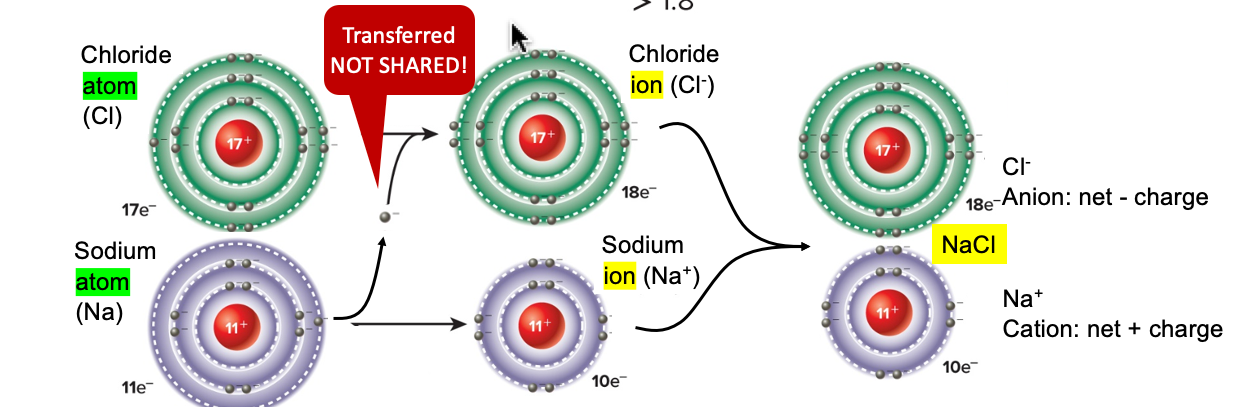
ion
an atom or molecule gained/lost one or more electrons
46
New cards
solvent
liquid
47
New cards
solute(s)
dissolved substances
48
New cards
solvent + solute(s) =
solution
49
New cards
water + hydrophilic molecules
aqueous solution
50
New cards
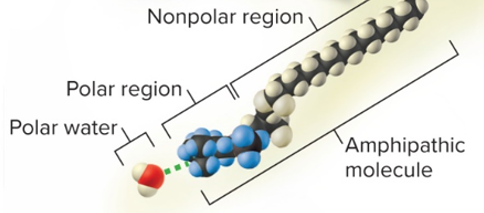
hydrophilic
water loving
\
solutes are molecules that are:
* ionic and/or with polar covalent bonds
\
solutes are molecules that are:
* ionic and/or with polar covalent bonds
51
New cards
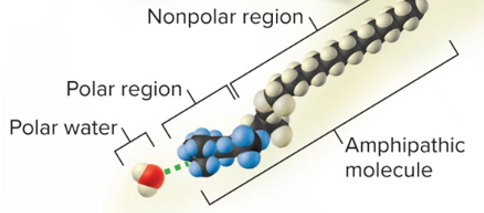
hydrophobic
water fearing
\
solutes are molecules that are:
* nonpolar like hydrocarbons, oils
\
solutes are molecules that are:
* nonpolar like hydrocarbons, oils
52
New cards
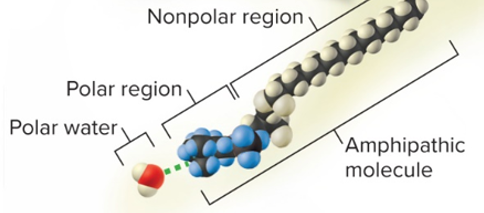
amphipathic
both hydrophilic and hydrophobic
\
solutes are molecules that are:
* both polar/ionized **and** nonpolar at the same time, like detergent
\
solutes are molecules that are:
* both polar/ionized **and** nonpolar at the same time, like detergent
53
New cards
cohesion (property of water)
water molecules stick to other water molecules
* hydrogen bonds make water molecules stick to one another
* hydrogen bonds make water molecules stick to one another
54
New cards
adhesion (property of water)
water molecules are attracted and stick to other substances
* hydrogen bonds allow water molecules stick to other substances
* hydrogen bonds allow water molecules stick to other substances
55
New cards
surface tension (property of water)
allows it to resist an external force, due to the cohesive nature of its molecules
* molecules at liquid surface attract each other
* molecules at liquid surface attract each other
56
New cards
capillary action (property of water)
the upward motion against gravity
* depends on the attraction between water molecules and the glass walls of the tube (adhesion), as well as on interactions between water molecules (cohesion)
* water molecules are more strongly attracted to the glass than they are to other water molecules (because glass molecules are even more polar than water molecules)
* depends on the attraction between water molecules and the glass walls of the tube (adhesion), as well as on interactions between water molecules (cohesion)
* water molecules are more strongly attracted to the glass than they are to other water molecules (because glass molecules are even more polar than water molecules)
57
New cards
acids
releases H+ in solution
* strong acids releases more H+
* ex: human stomach acid → pH= \~2
* ex: undiluted orange juice → pH= \~4
* strong acids releases more H+
* ex: human stomach acid → pH= \~2
* ex: undiluted orange juice → pH= \~4
58
New cards
bases
lowering the \[H+\] by releasing OH- and/or binding to H+
* ex: baking soda → pH= \~9
* ex: bleach → pH= \~12
* ex: baking soda → pH= \~9
* ex: bleach → pH= \~12
59
New cards
buffer
maintains a constant pH by removing H+ or releasing H-
60
New cards
molecules can be __ and ____
inorganic and organic
61
New cards
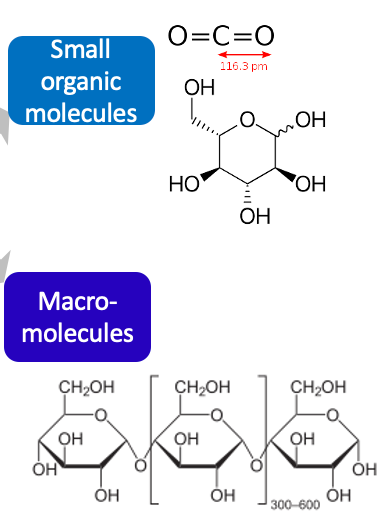
organic molecules
**contains carbon** that tends to be abundant in living organisms
\
* small organic molecules
* macromolecules
\
* small organic molecules
* macromolecules
62
New cards
vitalism
19th-century concept that organic molecules were created by and imparted with a vital life force within a plant or animal’s body
* believed organic compounds could not be synthesized
* later disproven—organic compounds can be synthesized
* believed organic compounds could not be synthesized
* later disproven—organic compounds can be synthesized
63
New cards
carbon contains how many electrons and how many valance electrons?
6 electrons and 4 valence electrons
64
New cards
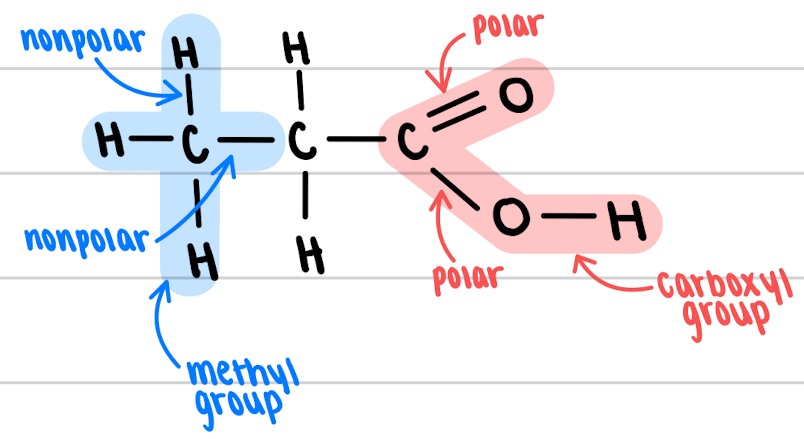
**how many bonds can carbon form** while the molecule created does not carry charges?
4
\
* can be polar or nonpolar bonds!!
* molecules with polar bonds are water-soluble
* molecules with nonpolar bonds (like hydrocarbons) are not very water soluble
\
* can be polar or nonpolar bonds!!
* molecules with polar bonds are water-soluble
* molecules with nonpolar bonds (like hydrocarbons) are not very water soluble
65
New cards
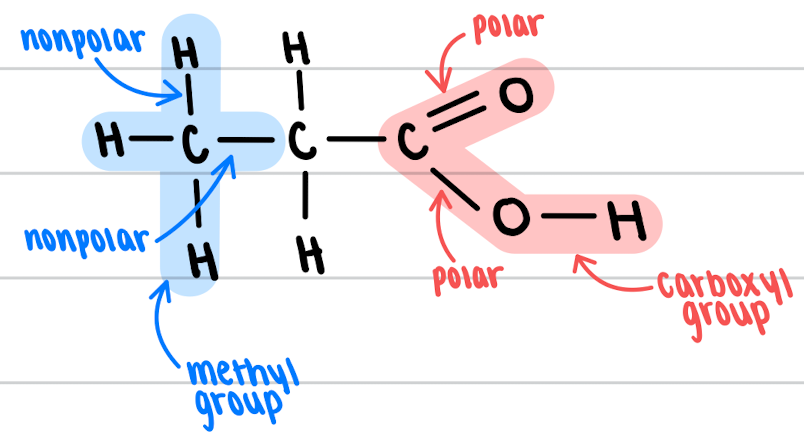
functional group
an atom or group of atoms within a molecule that has similar chemical properties whenever it appears in various compounds
\
* ex: methyl group
* ex: carboxyl group
\
* ex: methyl group
* ex: carboxyl group
66
New cards
isomers
molecules with identical molecular formula but different structures
* allows more diversity in chemical structure even though the atomic compositions are the same
* allows more diversity in chemical structure even though the atomic compositions are the same
67
New cards
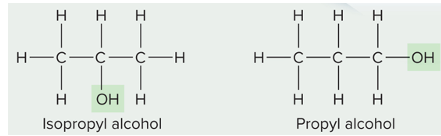
structural isomer
same atoms, different bonding relationships
68
New cards
stereo-isomer
identical bonding relationships, different spatial arrangements
* cis-trans isomers
* enantiomers
* cis-trans isomers
* enantiomers
69
New cards
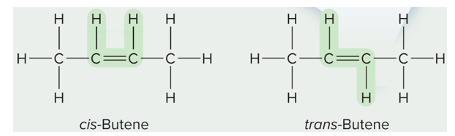
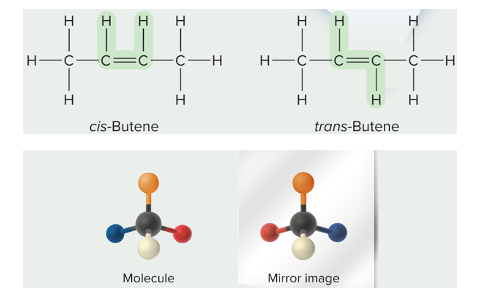
cis-trans isomers
different positioning around double bond __**or**__ rings
70
New cards
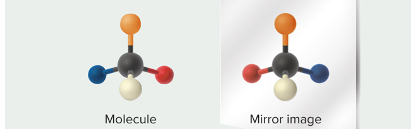
enantiomers
mirror image molecules
* difference in orientation leads to different binding abilities
* enzymes that recognize one enantiomers usually do not recognize the other
* difference in orientation leads to different binding abilities
* enzymes that recognize one enantiomers usually do not recognize the other
71
New cards
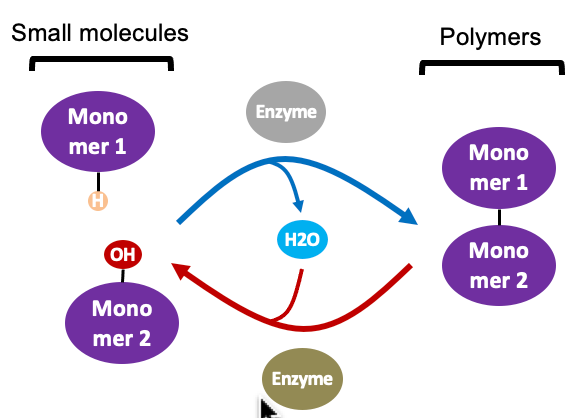
condensation/dehydration (top)
a molecule of **water is removed** each time a new **monomer is added** (that’s why it’s called a “dehydration” reaction)
* the process repeats to form long polymers
* a polymer can consist of thousands of monomers
* dehydration is catalyzed by enzymes
* the process repeats to form long polymers
* a polymer can consist of thousands of monomers
* dehydration is catalyzed by enzymes
72
New cards
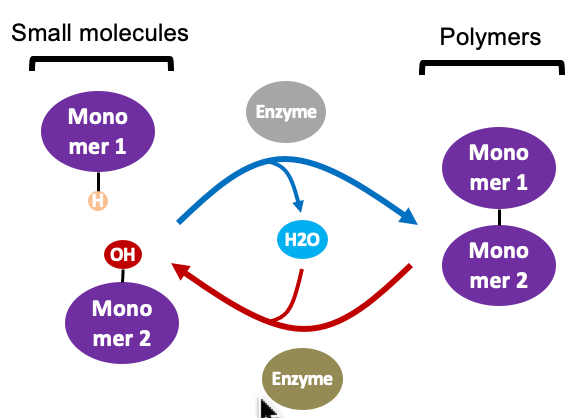
hydrolysis (bottom)
a molecule of **water is added** back each time a **monomer is released**
* the process repeats to break down a long polymer
* hydrolysis is catalyzed by enzymes
* the process repeats to break down a long polymer
* hydrolysis is catalyzed by enzymes
73
New cards
what are the four major types of organic molecules
DNA/RNA (nucleic acids), proteins, lipids, carbohydrates
74
New cards
3 properties of nucleic acids
storage, expression, and transmission of genetic information
75
New cards
3 components of a nucleotide
phosphate, 5 carbon sugar (either ribose or deoxyribose), base (single or double ring of carbon and nitrogen atoms)
76
New cards
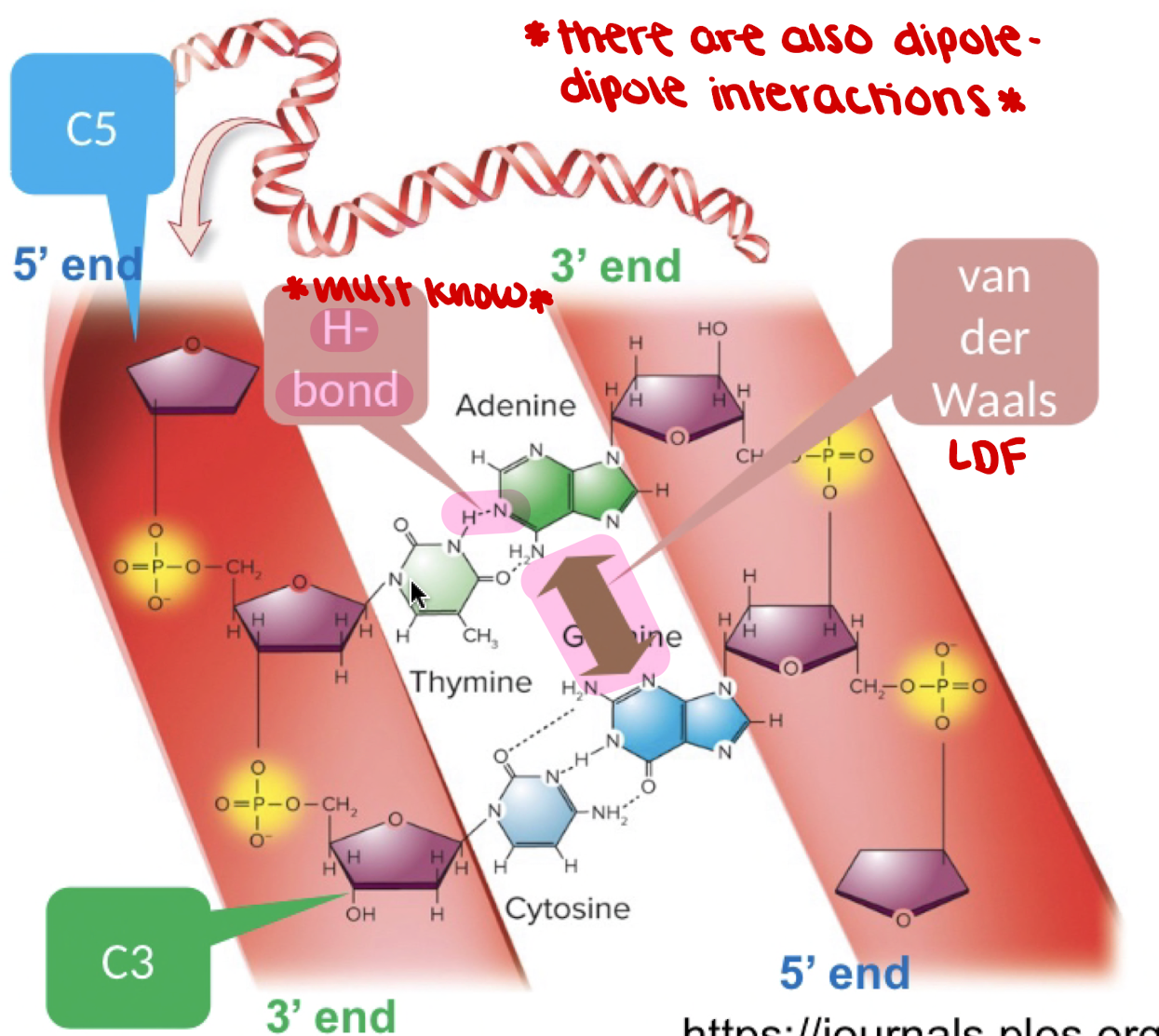
components of nucleotides in DNA
* phosphate
* sugar: deoxyribose
* base: cytosine
\
* double helix
* nucleotides: dA, dG, dC, dT (thymine)
* sugar: deoxyribose
* base: cytosine
\
* double helix
* nucleotides: dA, dG, dC, dT (thymine)
77
New cards
components of nucleotides in RNA
* phosphate
* sugar: ribose
* base: uracil
\
* single strand
* nucleotides: A (adenine),G (guanine),C (cytosine),U (uracil)
* sugar: ribose
* base: uracil
\
* single strand
* nucleotides: A (adenine),G (guanine),C (cytosine),U (uracil)
78
New cards
in DNA, adenine pairs with
thymine
79
New cards
in DNA, cytosine pairs with
guanine
80
New cards
purines
adenine (A) and guanine (G)
81
New cards
pyrimidines
cytosine (C) and thymine (T)
82
New cards
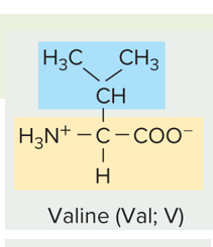
is this amino acid side chain polar (charged or un-charged) or non polar
nonpolar
83
New cards
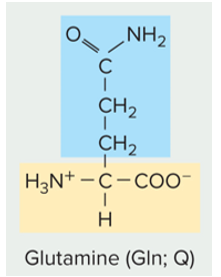
is this amino acid side chain polar (charged or un-charged) or non polar
polar un-charged
84
New cards
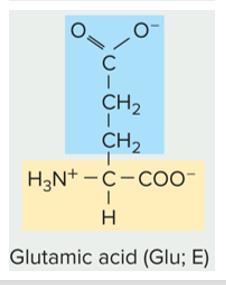
is this amino acid side chain polar (charged or un-charged) or non polar
polar charged
85
New cards
how do amino acids form polypeptides and proteins
amino acids are joined together by dehydration reactions
* chains of amino acids
* polymers of amino acids known as polypeptides
* proteins may be formed form one or several polypeptides
* chains of amino acids
* polymers of amino acids known as polypeptides
* proteins may be formed form one or several polypeptides
86
New cards
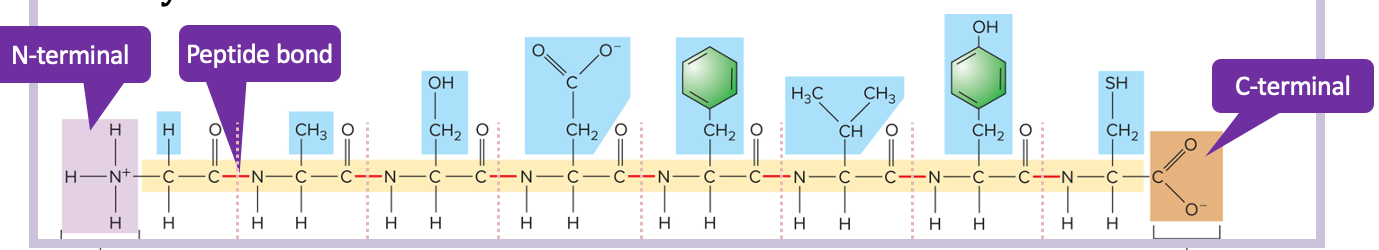
protein primary structure
* the free amino group of a polypeptide is the N-terminal
* the free carboxyl end is the C-terminal
* carboxy + amino forms peptide bond
* the free carboxyl end is the C-terminal
* carboxy + amino forms peptide bond
87
New cards
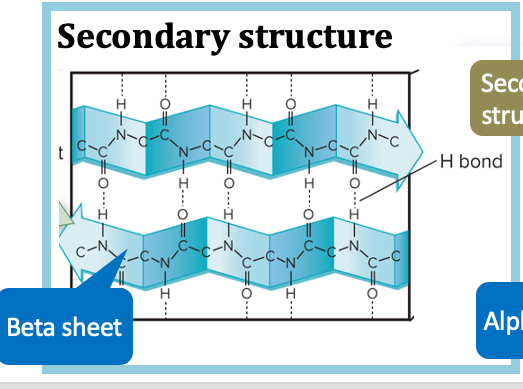
protein secondary structure
folded structures that form within a polypeptide due to hydrogen bonds between atoms of the backbone
88
New cards
gene expression (protein functions)
RNA polymerase: synthesize RNA using DNA as a template
\
polymerase: an __enzyme__ which brings about the formation of a particular polymer, especially DNA or RNA.
\
polymerase: an __enzyme__ which brings about the formation of a particular polymer, especially DNA or RNA.
89
New cards
motor (protein functions)
myosin: contractile force of muscles
90
New cards
defense (protein function)
antibodies: destroy pathogens
91
New cards
metabolism (protein function)
hexokinase: phosphorylate glucose
92
New cards
signal transduction
taste receptors: taste molecules in food
93
New cards
stucture (protein functions)
actin: shape to the cytoplasm of plant and animal cells
94
New cards
transporter (protein functions)
glucose transporters: move glucose from outside to inside cells
95
New cards
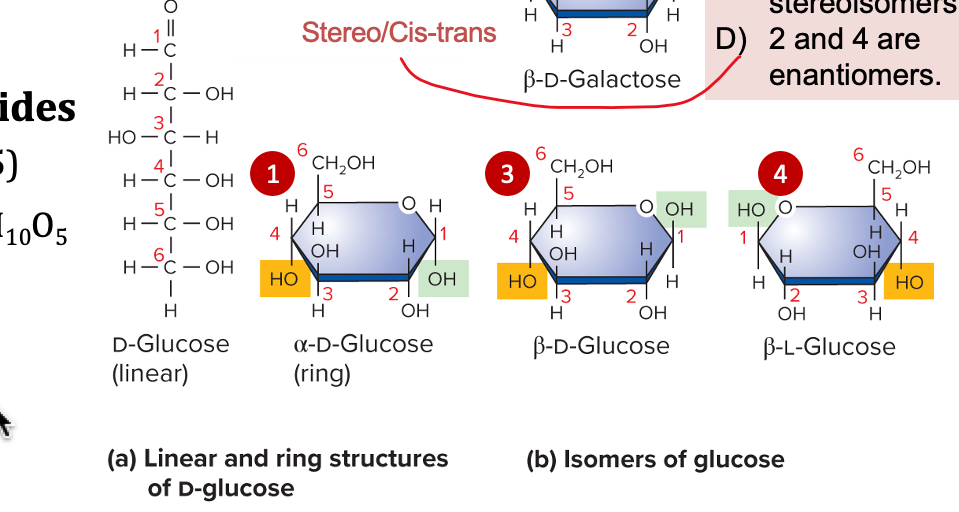
monosaccharides
one sugar molecule
96
New cards
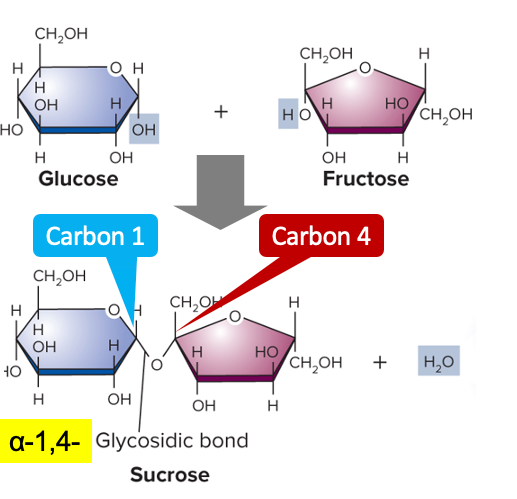
disaccharides
2 monosaccharides joined by dehydration/condensation
ex: sucrose, maltose, lactose
ex: sucrose, maltose, lactose
97
New cards
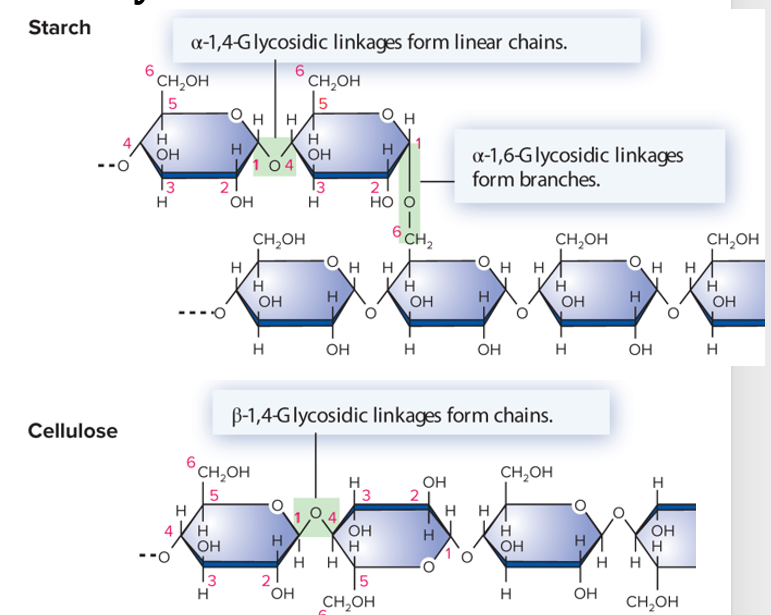
polysaccharides
many monosaccharides linked together to form long polymers
ex: starch & cellulose
ex: starch & cellulose
98
New cards
features of lipids
* composed of hydrogen, carbon, and some oxygen
* nonpolar, so very insoluable in water
* classes of lipids: fats, phospholipids, steroids, waxes
* comprises about 40% of the organic matter in the average human body
* nonpolar, so very insoluable in water
* classes of lipids: fats, phospholipids, steroids, waxes
* comprises about 40% of the organic matter in the average human body
99
New cards
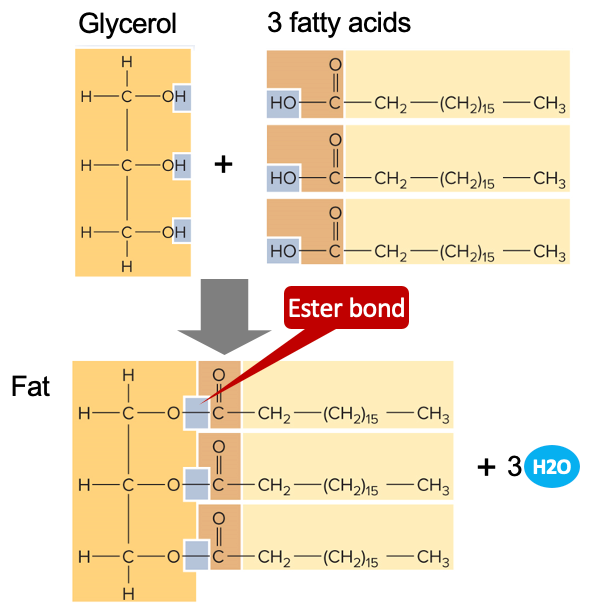
fats (a class of lipid, also called triglycerides)
* formed by bonding glycerol to 3 fatty acids
* joined by dehydration; resulting bond is an ester bond
* important for energy storage
\
fatty aids:
* saturated
* unsaturated
* cis
* trans
* joined by dehydration; resulting bond is an ester bond
* important for energy storage
\
fatty aids:
* saturated
* unsaturated
* cis
* trans
100
New cards
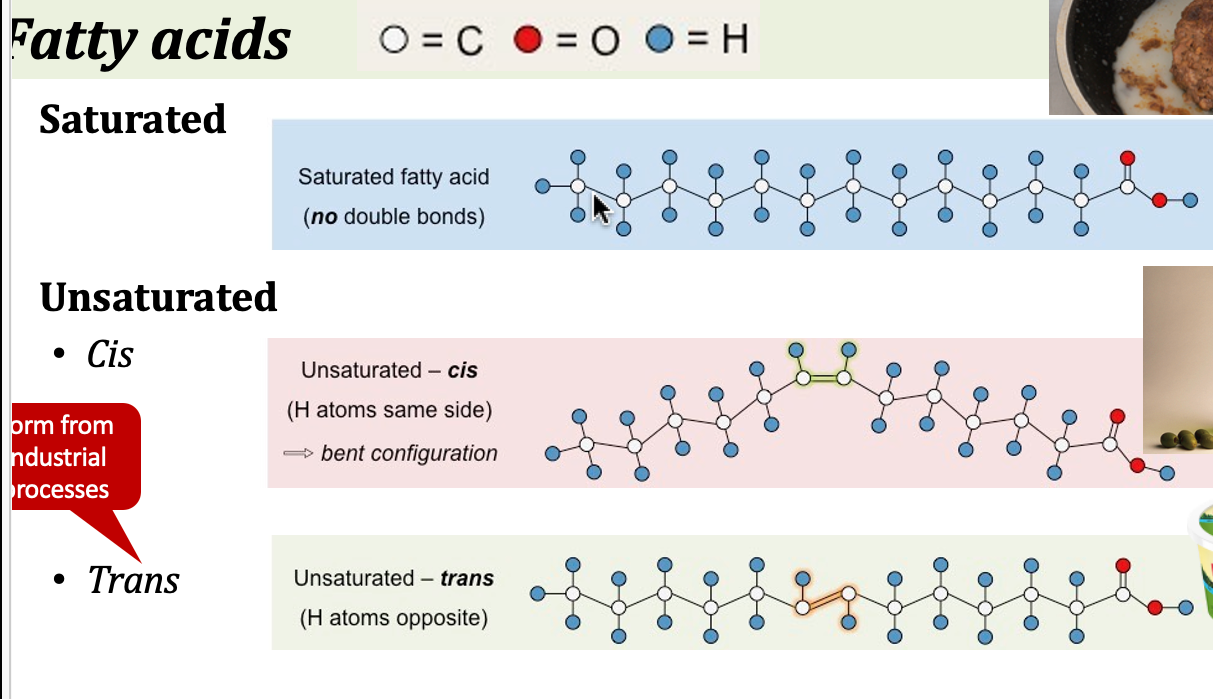
saturated fatty acids
all carbons have the maximal amount of hydrogens
* tend to be solid at room temperature
* tend to be solid at room temperature