1.5. CHEMICAL ENERGETICS
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
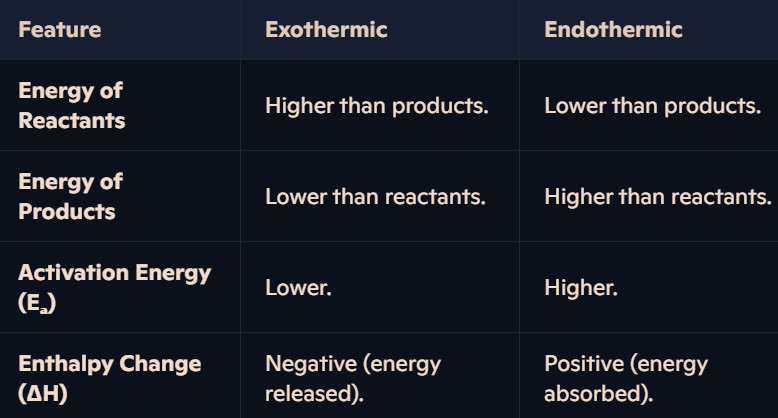
What are the differences between exothermic and endothermic reaction diagrams?
What are standard conditions (⦵)?
Definition: Standard conditions refer to:
Pressure: 101 kPa.
Temperature: 298 K (25°C).
Substances in their normal physical states (solid, liquid, or gas).
Symbol: Reactions carried out under standard conditions use the symbol θ (e.g., ΔHθ).
What are the key types of enthalpy changes and their definitions?
Standard Enthalpy Change of Reaction (ΔHθr):
The enthalpy change when reactants in the stoichiometric equation react to form products under standard conditions.
Can be exothermic or endothermic.
Standard Enthalpy Change of Formation (ΔHθf):
The enthalpy change when one mole of a compound is formed from its elements under standard conditions.
Can be exothermic or endothermic.
Standard Enthalpy Change of Combustion (ΔHθc):
The enthalpy change when one mole of a substance is burned in excess oxygen under standard conditions.
Always exothermic.
Standard Enthalpy Change of Neutralisation (ΔHθneut):
The enthalpy change when one mole of water is formed by reacting an acid and an alkali under standard conditions.
Always exothermic.
Why do energy transfers occur during chemical reactions?
During a reaction, bonds in reactants are broken and new bonds in products are formed:
Bond Breaking:
Requires energy from the surroundings → endothermic (+ΔH).
Bond Forming:
Releases energy to the surroundings → exothermic (–ΔH).
Overall Reaction Enthalpy:
Endothermic Reaction: More energy is required to break bonds than is released when forming bonds.
Exothermic Reaction: More energy is released when forming bonds than is required to break bonds.
What are exact and average bond energies?
Exact Bond Energy (Bond Dissociation Energy):
Energy required to break one mole of a specific covalent bond in the gas phase.
Example: E(H–H) is the bond energy of single bonds between two hydrogen atoms.
Average Bond Energy:
The average of the same type of bond (e.g., C–H) in different molecules.
Bond energies vary slightly due to the chemical environment of atoms.
How are enthalpy changes (ΔH) calculated using bond energies?
Formula: ΔHθr = (Energy required to break bonds) + (Energy released to form bonds).
Sign Convention:
Values for bonds broken are positive (endothermic).
Values for bonds formed are negative (exothermic).
How is enthalpy change measured experimentally?
Technique: Calorimetry using equipment such as a polystyrene cup.
Formula for Heat Transfer:q=m×c×ΔT, where:
q: Heat transferred (J).
m: Mass of water (g).
c: Specific heat capacity (4.18 J g⁻¹ K⁻¹ for water).
ΔT: Temperature change (°C or K).
Enthalpy Change (ΔH): ΔH=−q/n, where:
n: Number of moles of the reactant or product.
If ΔH is negative, the reaction is exothermic.
If ΔH is positive, the reaction is endothermic.
How does Hess's law apply to calculate enthalpy change of reaction (ΔHθr)?
Equation: ΔHr = ΔH2 – ΔH1, where:
ΔH2 = Direct enthalpy change from elements to products.
ΔH1 = Indirect enthalpy change from elements to reactants.
The enthalpy change from elements to products (direct route) is equal to the enthalpy change of elements forming reactants and then products (indirect route)
Equation
ΔH2 = ΔH1 + ΔHr
Therefore,
ΔHr = ΔH2 – ΔH1

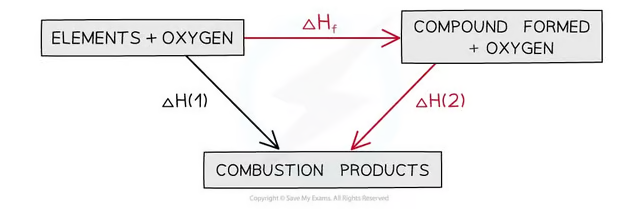
How does Hess's law apply to calculate enthalpy change of formation (ΔHθf)?
Equation: ΔHf = ΔH1 – ΔH2, where:
ΔH1 = Combustion of the compound.
ΔH2 = Combustion of elements forming the compound.
The combustion products can be formed directly from elements to combustion products = ΔH1 OR The combustion products can be formed indirectly from elements to compounds to combustion products = ΔHf + ΔH2
The enthalpy change going from elements to products (direct route) is equal to the enthalpy change of elements forming reactants and then products (indirect route)
Equation
ΔH1 = ΔHf + ΔH2
Therefore,
ΔHf = ΔH1 – ΔH2
How are average bond enthalpies calculated using Hess’s law?
Steps:
Write the equation for bond dissociation.
Use relevant enthalpy values: atomisation and formation.Bond energies cannot be found directly so enthalpy cycles are used to find the average bond energy
This can be done using enthalpy changes of atomisation and combustion or formation
The enthalpy change of atomisation (ΔHθat ) is the enthalpy change when one mole of gaseous atoms is formed from its elements under standard conditions.
E.g. ΔHθat [H2] relates to the equation:
½H2 (g) → H (g)