my own bonding 2
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
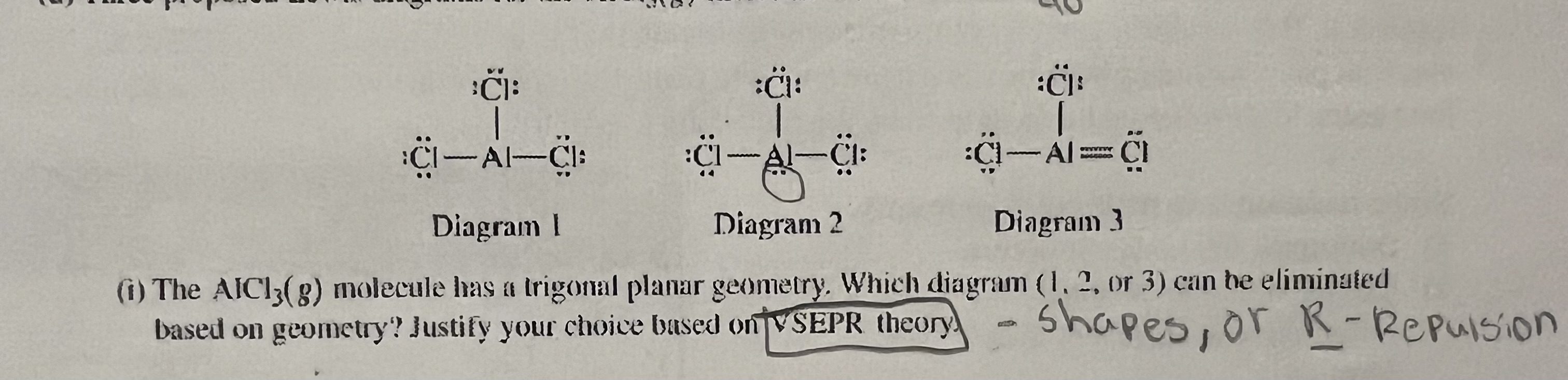
Diagram 2. The lone pair on the Al makes it a different shape than trigonal planar, which has 3 electron domains not 4.
Justify based on VSEPR theory
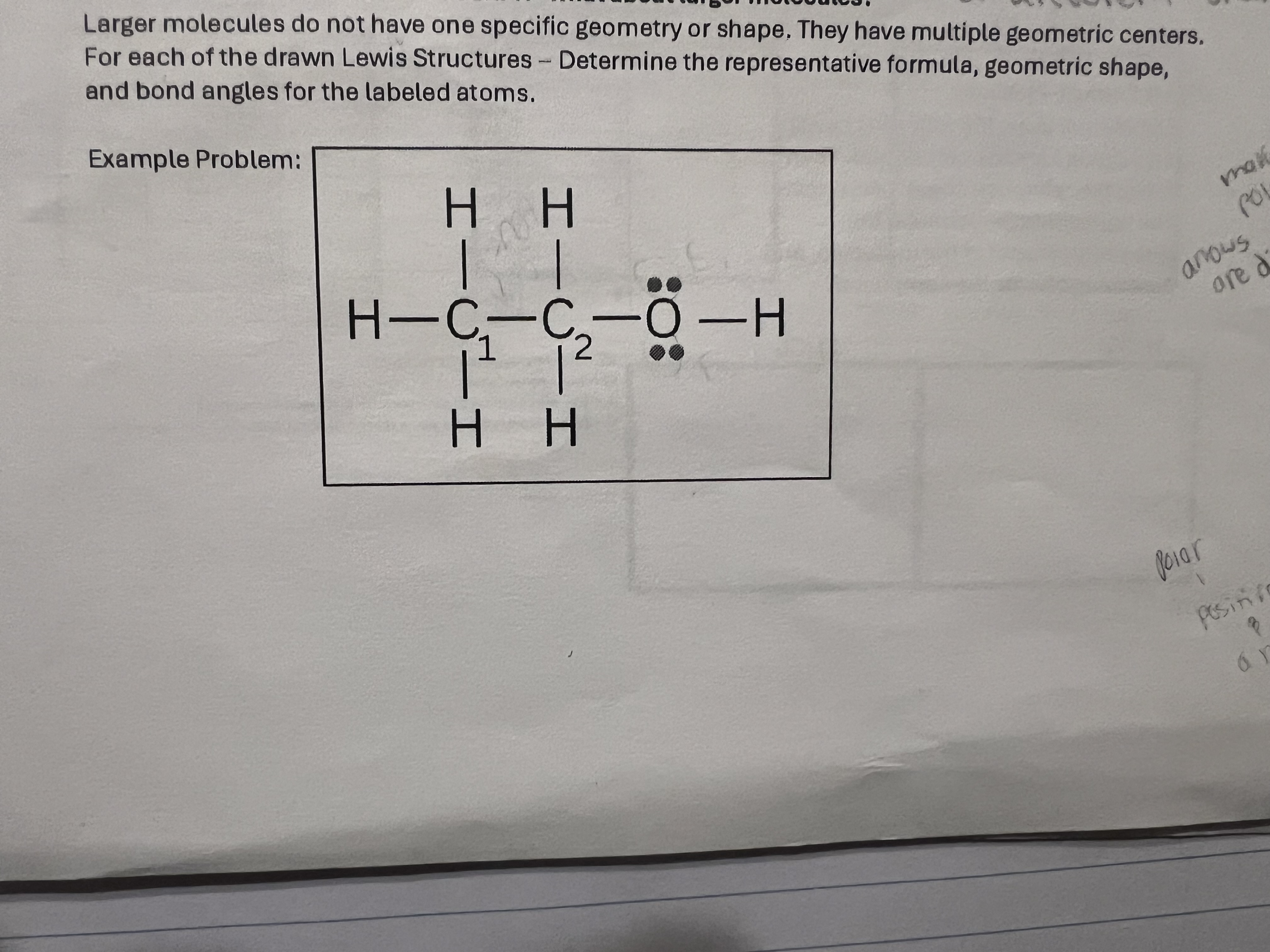
C1- tetrahedral - 109
C2 - same
O - bent 105
Answer shape and angle for C1 , C2, and O
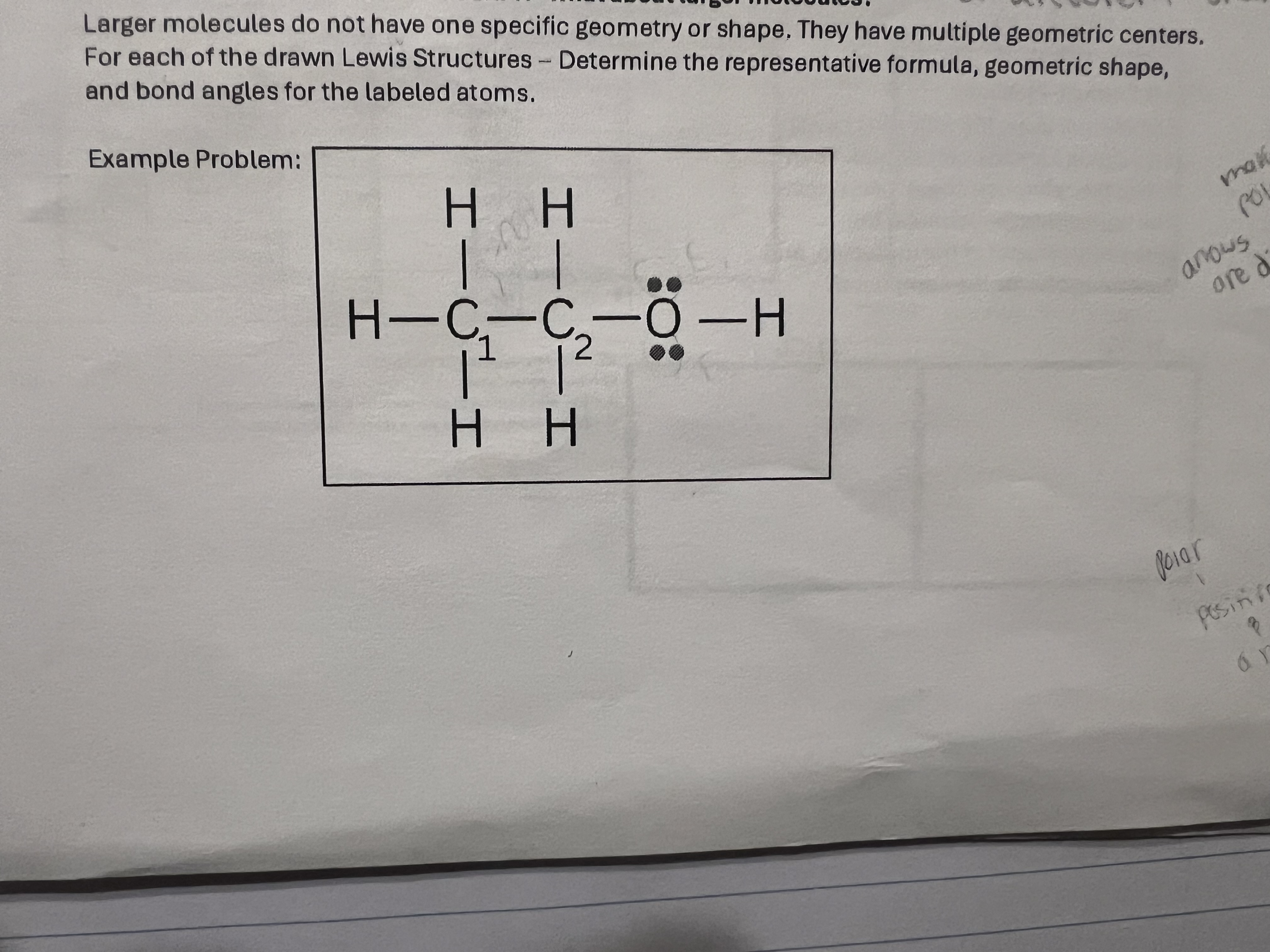
C1-sp3
C2- same
O-sp3
Answer hybridization for C1, C2, and O
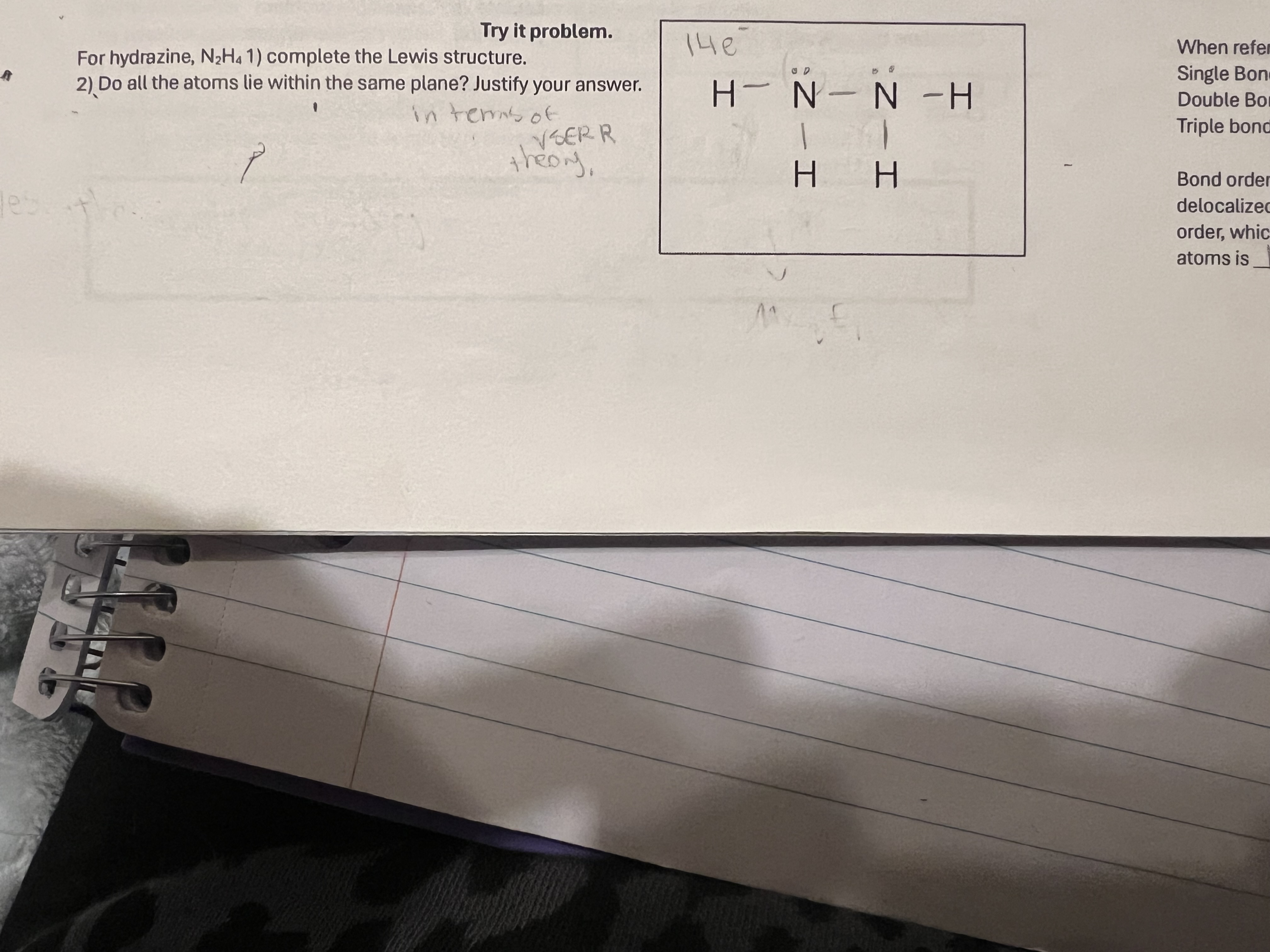
No, both N atoms follow trigonal pyramidal, so they do not fall on the same plane.
Answer
Linear
Trigonal planar
Bents
Square
Four types that DO fall on the same plane
The distance in pm between nuclei in a bond
Bond length
The balance between the attractive and repulsive forces
On point x on the graph, marks
A single
bond has fewer electrons being shared, so the attraction is weaker. The atoms aren't pulled as tightly together, resulting in a longer distance(internuclear distance) between them.
Why are single bonds longer than double bonds MOST OF THE TIME
The more shared electrons create a stronger attractive force between the atoms. This pulls the atoms closer together.
Why are double and triple bonds short?
Two atoms share one pair of electrons.
Single bond
Two atoms share two pairs of electrons.
Double Bond
Two atoms share three pairs of electrons.
Triple Bond
Br has electrons on 4 energy levels (n=4) while F only has electrons on 2 (n=2). The extra shells from Br increase the distance between the H and Br nuclei, giving HBr the greater bond length.
Based on the arrangement of electrons in the Br and F atoms, explain why the bond length and an HBr molecule is greater than that an HF molecule
Energy required(absorbed) to break a bond in Kj/Mol
Bond strength/energy
Shorter bond lengths
Bonds with H atoms tend to have
Bond energy
Bond length
The y axis is ____
The x axis is ____
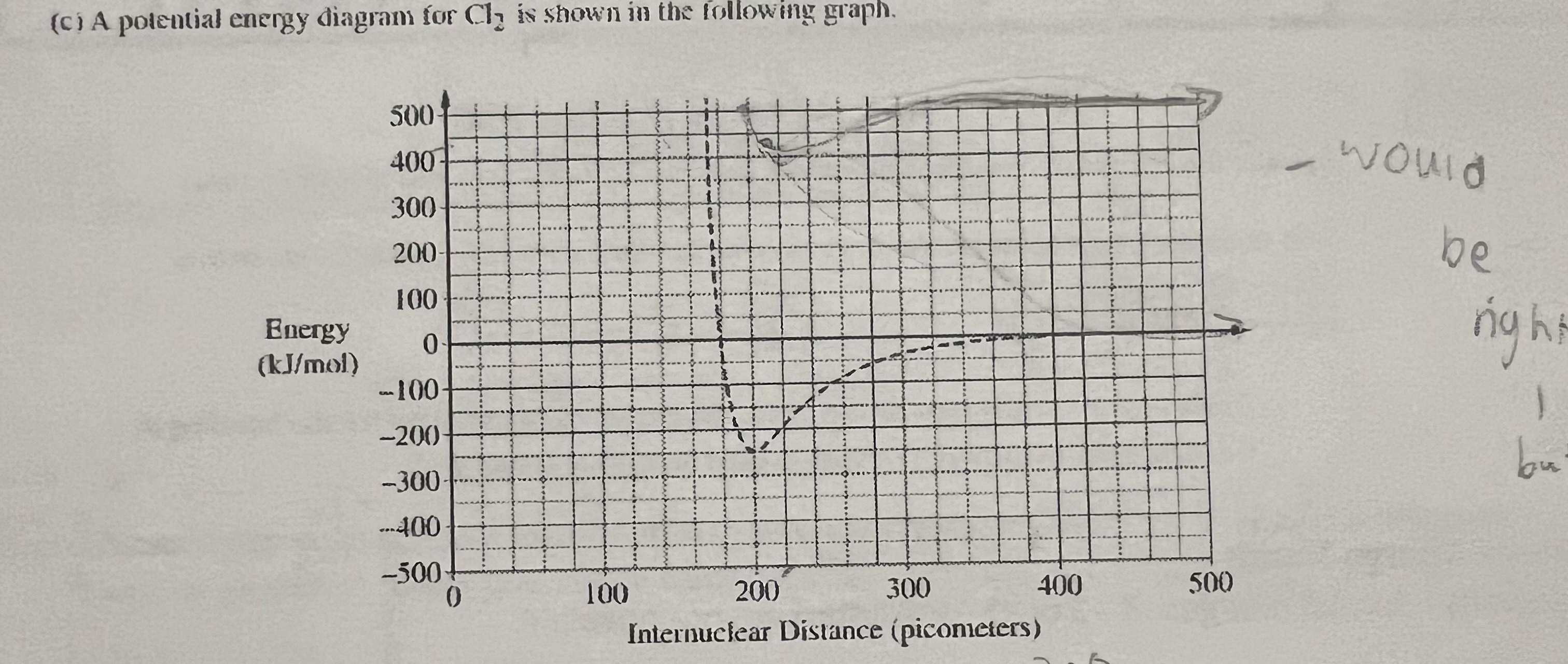
positive or negative despite the way it is in the duration
On a curve frq that asks you to draw, the energy in kj/mol can be written as .
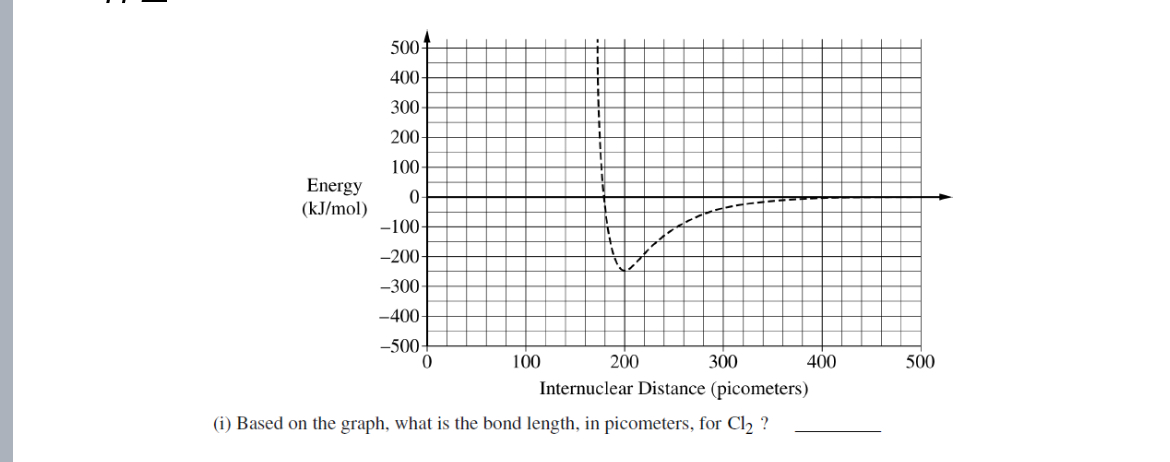
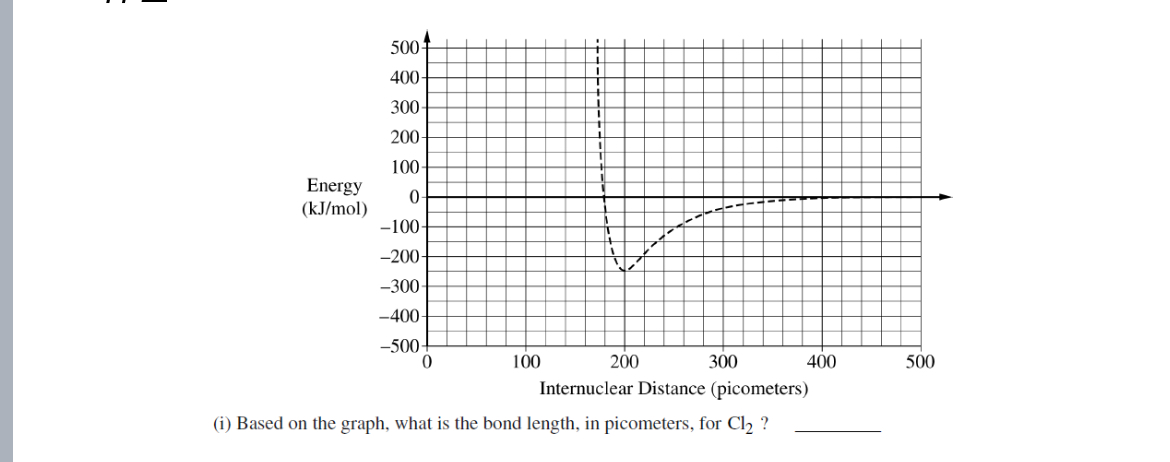
200 pm
Based on the graph, what is the bond length in pico meters for Cl2?
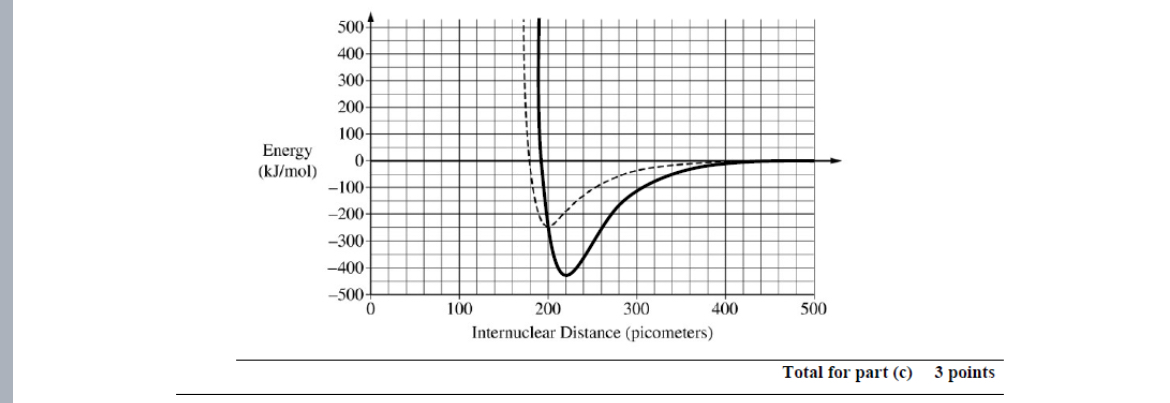
A student finds that the average bond length is 220 pico meters in the average bond energy is 425 Kj/mol. Draw the potential energy curve for the average bond found by the student.
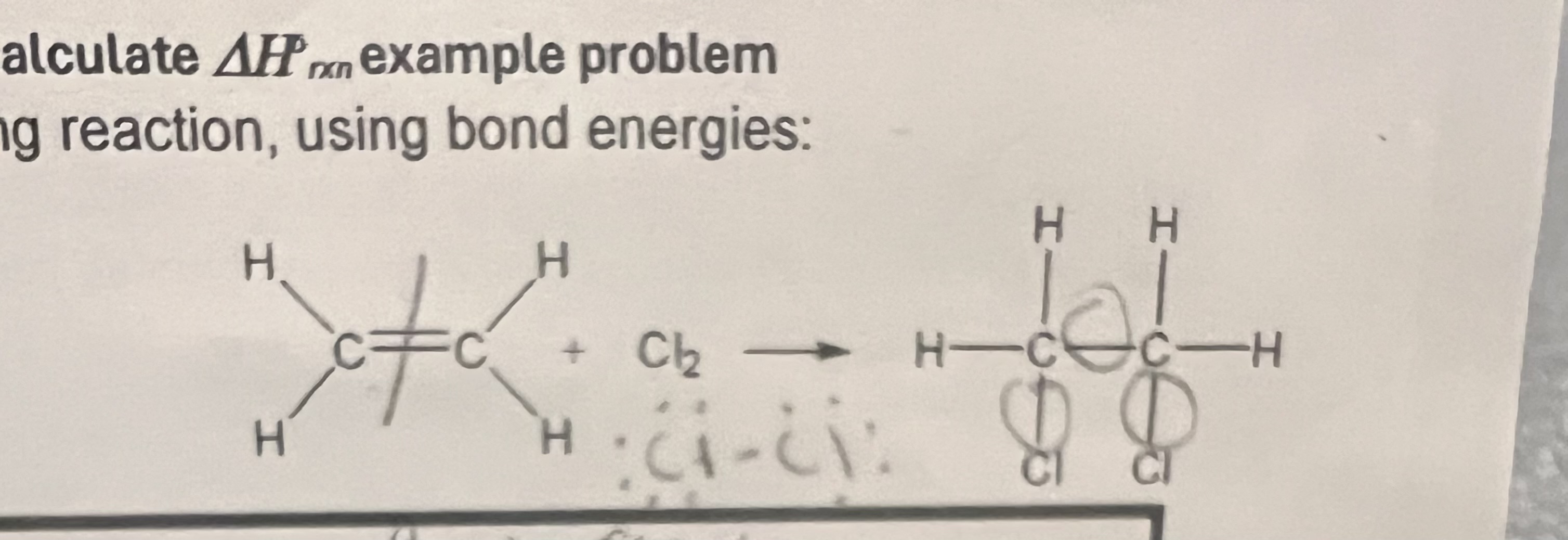
Triple bond
Which of these 3 bonds will have the greatest bond strength(energy) in pm or Kj/mol?

Broken: 1 C=C
1 Cl-Cl
Formed: 1 c-c
2 C-Cl
How many bonds formed and broken?
Kj of bonds formed - Kj of bonds broken
For bond energies to calculate deltaHrxn the formula is
Bonds broken for a negative enthalpy
Bonds formed has to be bigger than
A large negative delta h/enthalpy results from
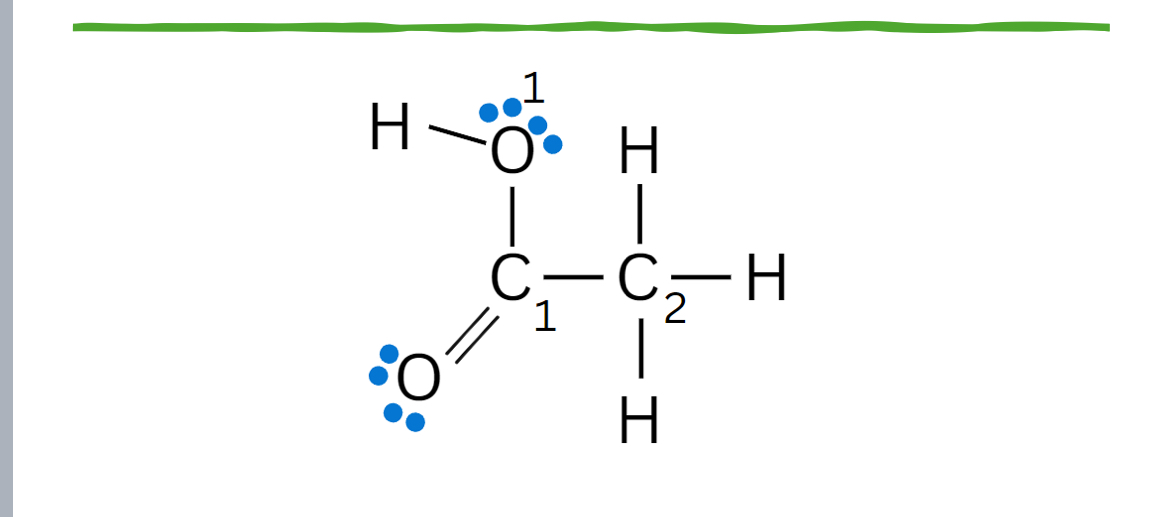
Sp2
Sp3
Sp2
Hybridization of the C1, C2 and bottom O?

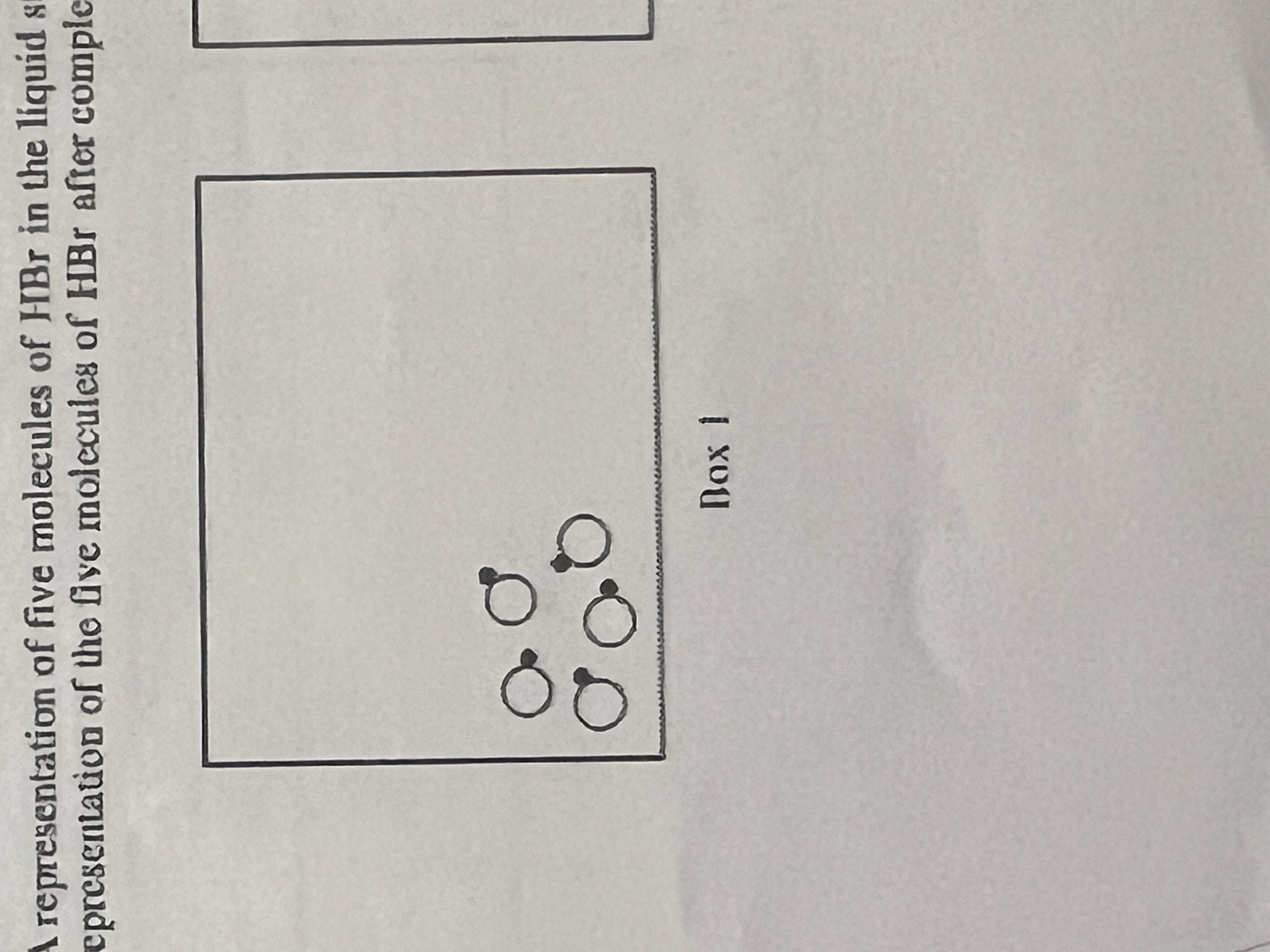
Draw in another box what the five molecules of HBr would look like AFTER vaporization has occurred
Gas to liquid
Condensation
Solid to gas
Sublimination
Solid to liquid (melt)
Fusion
mcdeltaT
q=
More London dispersion bc there is a higher chance of electrons being unevenly distributed
More electrons mean
Ionic compounds have highest boiling points - strongest intermolecular forces
How to identify compound by boiling point?