Electronic Structure of Matter
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
Electron
Discovered by Sir Joseph John Thomson in 1897, it is a subatomic particle with a negative charge.
Anode
The positively charged electrode in a battery or an electric cell.
Bohr's model
Niels Bohr's model of the atom, which introduced the concept of orbits or energy levels where electrons can be found.
Energy levels
Stable discrete regions in Bohr's model where electrons do not experience spontaneous energy absorption and emission.
Quantized energy levels
In Bohr's model, each energy level has a definite amount of energy, and the amount of released energy in the form of light is equivalent to a specific wavelength.
Quantum mechanical model
A model developed by Louis de Broglie, which describes electrons as waves or electron clouds surrounding the nucleus, and denies the probability of having electrons in definite orbits.
Orbitals
Regions in the quantum mechanical model where electrons are most probably found.
Schrödinger equation
A mathematical equation used to compute the orbitals in the quantum mechanical model.
Quantum numbers
Four numbers that describe the unique set of characteristics of an electron in the quantum mechanical model.
Pauli's exclusion principle
The principle that states that no two electrons can have the exact same set of quantum numbers, and electrons in the same orbital must have opposite spins.
Aufbau principle
The principle that atomic orbitals are filled from the lowest energy to the highest energy.
Hund's rule
The rule that states that every orbital in the same subshell must be filled singly before being paired, and electrons in singly occupied orbitals should have parallel spins.
Nucleus
In 1911, Lord Ernest Rutherford's discovered the _______
1803, Solid Sphere Model
In ____, John Dalton proposed the ___________ of the atom.
Nuclear Model
Ernest Rutherford proposed a new model which he called the _______ _____ of the atom.
atomos
Greek word for “atom”
Werner Heisenberg
He made the uncertainty principle which says “we cannot know both the position and speed of a particle, such as a photon or electron, with perfect accuracy”
Uncertainty Principle
“we cannot know both the position and speed of a particle, such as a photon or electron, with perfect accuracy”
Principal Quantum Number
It refers to the distance of the electron from the nucleus, it is also known as the shell.
Azimuthal Quantum Number
It describes the shape of the atomic orbital, it is also known as the subshell
Each value of ℓ corresponds to a subshell shape
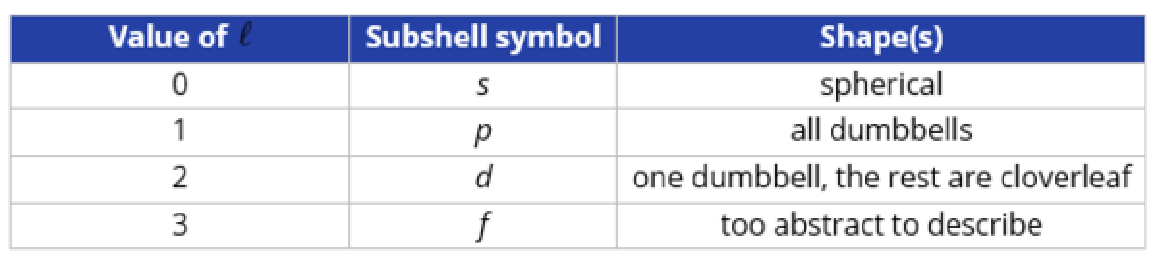
Magnetic Quantum Number
The number of orbitals in a subshell.
The values of mℓ are ℓ to +ℓ including zero.
Electron Spin Quantum Number
It indicates the spin of the electron or the direction at which it revolves around the nucleus.
The values of ms are +½ or ½ only.