organic chem
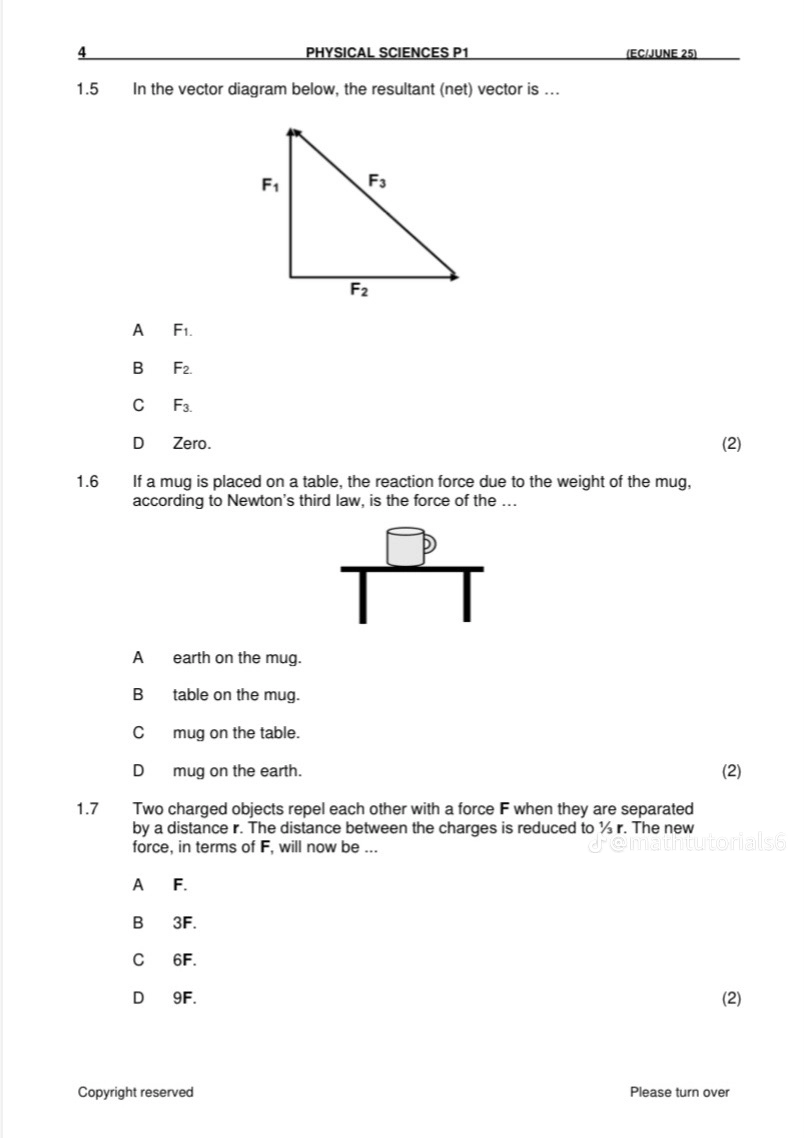
1/20
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
what is an organic molecule
A- An organic molecule is one which contains carbon (C) atoms. These are generally bonded to other carbon atoms as well as hydrogen (H) atoms. Other elements like oxygen (O), the halogens (F, Cl, I, Br), nitrogen (N), sulphur (S) and phosphorus (P).are common in these molecukes
T- These molecules range in size from simple molecules to complex structures containing thousands of atoms.
C- carbon is the building clock of all organic compounds and recycles through earths air soil water and living organisms
what is structural isomer
structural isomers as compounds having the same molecular formula but different structural formulas
What molecules contain carbon, but in organic
carbon monoxide (CO)
CARBONATES( HCO)
Bicarbonates
cyanides KCN) (CN)
Carbides (Molecules that contain carbon and a more literal negative element (CaC)
pure carbon compound like diamonds and graphite
what are some properties of carbon? atoms
C-Carbon has four valence electrons ( in a tetrahedral arrangement) : that each carbon atom can form up to 4 bonds with other atoms. including other carbon atoms
C- Carbon linkage: can result in long chains of carbon atoms,
which can be 1) straight(unbranched 🎋) - 2) - branched or —3)—cyclic .
Because of the number of bonds carbon conform with other atoms, carbon compounds can be very complex
C-CATENATION— is the process where by a chemical element bonds with itself to form, long chains or ring 💍 like structures . The ability of carbon atoms to form —> molecules, with long chains is called catenation.⛓
C-cracking : breaking long carbon chains —> into smaller carbon chains
carbons have the ability to form (l )double (ll ) or triple( lll) bond. The bonds formed between carbon and itself and other elements are strong covalent bonds.The ENΔ between C—H molecules is so small 🤏 that C- H molecules are almost pure covalent making most organic compounds Non-polar
What is structural formula?
structural formula indicates which atoms are attached to which within molecule atoms, atoms ⚛ are represented with their chemical symbol and lines are used to represent each bond that holds thes atoms ⚛ tgt
What is condensed structural formula?
show the way in which atoms bonded within molecules But Does not show all the bond lines -
When bonds between C Atoms are shown — but not all the C-H Atoms it is semi Condensed structural formula
condensed structural formula show each C- atom and H atom that are bonded as molecular formula
molecular formula
this is a chemical formula that represents the —type of atom & the correct number of each found in a molecule
the numbers are written in subscript after the ATOMIC SYMBOL
Functional group
an atom or group of atoms the form the center of reactivity within a molecule
homologous series
a series of similar compounds— which have the same functional group and same general formula . in which each member differs from the previous by a single 1) CH unit
ALKANE
Saturated HYDROCARBON- only C-C sb and C-H sb
ALKENE
UNSATURATED hydrocarbons C-H sb and C-C double bonds
ALKYNE
C-C triple bonds (unsaturated)
HALOALKANES
halogen atom bonded to saturated carbon atom CnH2n+1X
Alcohol
Hydroxyl group bonded to saturated carbon atom CnH2n+1(OH)
HYDROCARBONS
compounds which only contain Carbon and Hydrogen atoms
Saturated hydrocarbon
is a compound in which all bonds between carbon Atoms are single bonds
each Carbon atom bonds a maximum of 4 Other atoms .
they contained that maximum possible of hydrogen atoms
These are very stable and don’t react easily
Unsaturated hydrocarbons
A compound where there is at least one db/tp between carbon atoms ⚛
difference between saturated vs unsaturated
an unsaturated carbon atom may only be bonded with 2/3 other atoms ⚛
They N# of hydrogens > saturated hydrocarbon with the same N# of Carbons
They are less stable ( more reactive ) than saturated hydrocarbons
→ for unsaturated compounds today become stable one double /triple bond must be broken and additional atoms must be added