1. Principles of Electrochemistry
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
What is electrochemistry based on?
Redox reactions
What is oxidation?
The loss of electrons by a species; an increase in oxidation number; an increase in oxygen
What is reduction?
The gain of electrons; a decrease in oxidation number; decrease in oxygen/increase in hydrogen
What is an oxidizing agent?
An electron acceptor species that gets reduced.
What is a reducing agent?
An electron donor species that is oxidized.
What is oil rig?
Oxidation is Loss (of electrons)
Reduction is Gain (of electrons)
Place a strip of zinc into a Cu2+ solution and what observations allow you to determine the oxidising and reducing agent?
a spontaneous redox reaction will occur and Copper will be deposited onto the zinc metal strip
Therefore zinc is oxidised and is the reducing agent with the equation Zn(s) = Zn2+ (aq) + 2e-
Cu2+ is reduced and therefore is the oxidising agent with the equation: Cu2+(aq) + 2e- = Cu(s)
Remember oil rig = oxidation is the loss of electrons, reduction is gain.
How is a galvanic or voltaic cell constructed?
the linkage of two half cells
A current can be produced by separating the oxidising and reducing agent so that electrons can transfer through an external wire.
For example: a Zn metal piece attached to the external wire sits in Zn2+ aqueous solution and the other cell is copper metal in a solution of Cu2+ aqueous solution
A salt bridge also connects the two aqueous solutions to maintain the cation-anion balance between compartments
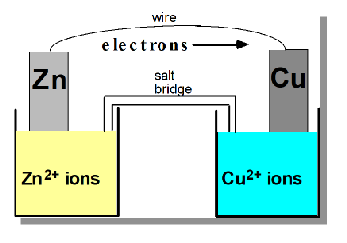
At which electrode does oxidation and reduction occur respectively?
Oxidation occurs at the negative anode
Reduction occurs at the positive cathode
Electrons travel through the external wire FROM the anode TO the cathode
Where do anions and cation travel across the salt bridge?
anions will travel toward the anode
cation will travels towards the cathode
salt bridge maintains the balance of the two ions in compartment
What is a cell diagram?
shorthand way to show the cell
Reduction is always on the right side of the diagram
anode on left, cathode on right

What is the Electromotive force, Ecell?
the cell voltage or cell potential
What is the standard cell potential?
the standard cell potential, Eo is the quantitative measure of the tendency of reactants to proceed to products when all are in their standard states at 25oC
tendency for a reduction process to occur at an electrode when all ionic species are present at 1M, all gases are at 1 bar/1 atm and when no metallic substance is indicated the potential is established with an inert metal electrode like Pt
What is the equation to find the cell potential at standard conditions?
Eocell = Eored (cathode) - Eoox (anode)
reduction - oxidation
E.g. Copper and zinc cell:
Copper has a Eo of +0.34V
Zinc is -0.76V
Therefore, Eocell = +0.034 - (-0.76) = +1.10V
the more negative E value is always the oxidised compound that is subtracted from the more positive value as E is a measure of the voltage DIFFERENCE
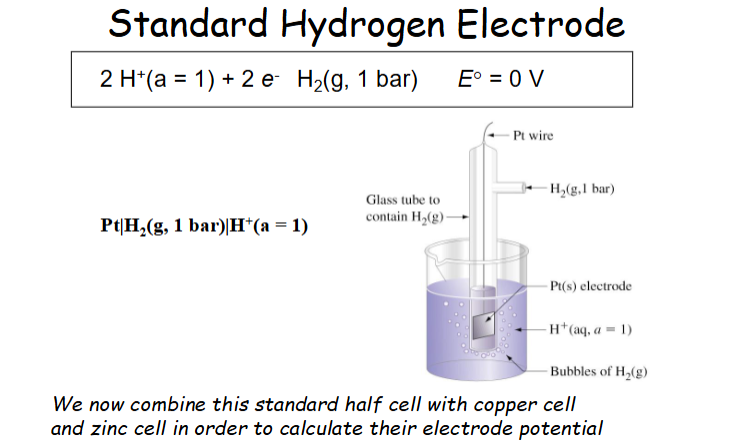
What is the standard hydrogen electrode?
the arbitrary zero that is taken for standard electrode potential
the difference in voltage for all other compounds was taken using hydrogen as the 0 mark.
How does stoichiometry affect Eo?
stoichiometry does not affect the value for the half cell
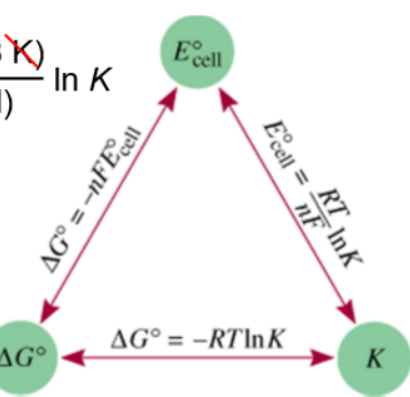
What is free energy equation?
ΔG = -nFE
Where n is the moles of electrons transferred, F is the Faraday constant (895000 C/mol)
Or ΔG= -RTlnK
What is the equation Triangle that relates E, ΔG and K?
ΔG - -nFE
E = RT/nF lnK
and ΔG = -RTlnK
What is the Nernst Equation?
E = E - RT/nF ln Q
Or it can become E = E - 0.0257/n lnQ or E = E - 0.059/n logQ
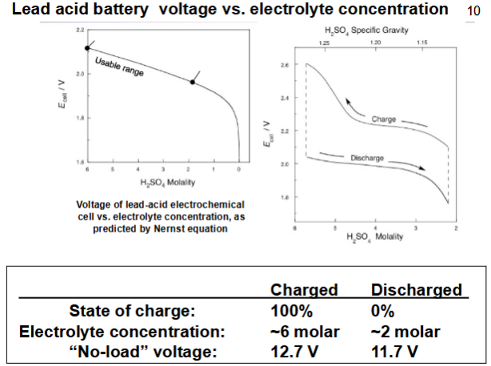
How can then Nernst equation and an accompanying graph sketch explain the useful operating range of the lead-sulphuric battery?
The Nernst equation will tell you the real voltage of the battery using E = Eo - 0.0592/n log Q
log Q is the reaction quotient
A graph of Ecell (in volts that has been calculated with the Nernst equation) vs H2SO4 molality can be taken and the usable range comes before the curved decline in voltage, that is the useful operating range for a lead-acid
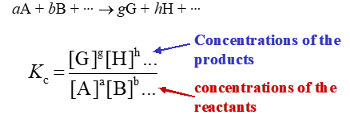
What is Q?
The reaction quotient, the ration of actual concentration of products/reactants
The same as Kc but not at standard conditions.
What is the activity coefficient? What can affect the activity coefficient value?
The activity coefficient, γ, relates the activity to concentration using the equation [a] = γ [C]
Where c is the nominal concentration of the substance
As C tends to 0, γ tends to 1
And as γ approaches 1, the value for a approaches C.
the value for γ can depend on concentration of ions and solution charge, ion charge and the diameter of the ion.
What is the equation for ionic strength?
Where I is the ionic strength
mi is the concentration of each ion in moles per L
Zi is the charge of the ion
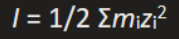
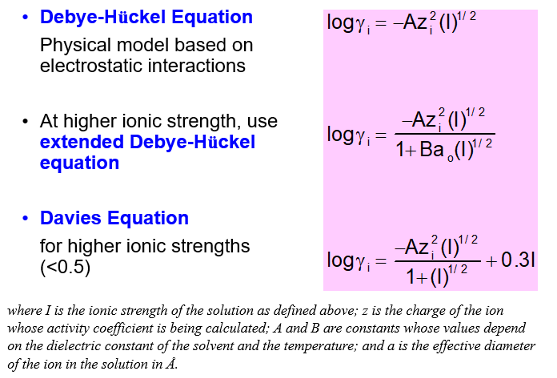
What is the Debye-Huckel equation?
A physical model based on the electrostatic interactions
I is the ionic strength of the solution
z is the charge of the ion that is having its activity coefficient calculated
A and B are constants whose value depend on the dielectric constant of the solvent and temperature
a is the effective diameter of the ion in the solution in A
For higher ionic strengths, the extended Deby-Huckel equation is used
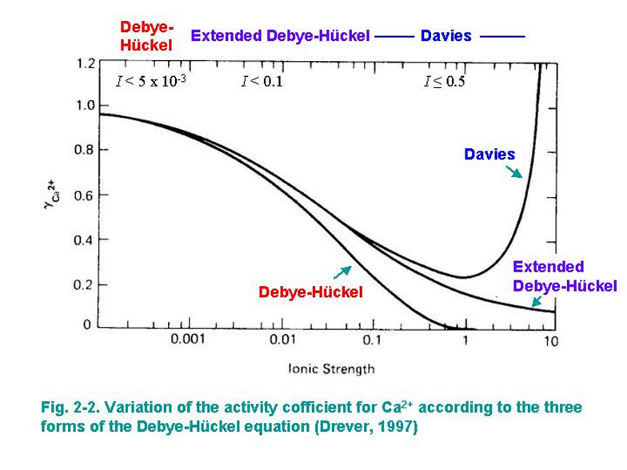
What is a general graph sketch of the Deby-Huckel and Davies equation?
They all start at the same point and the Debye-Huckel decreases quicker as ionic strength increases and hits 0 for the activity coefficient around 1
Extended Debye-Huckel decreases at a lower rate
Davies decreases with extended Debye-Huckel and after an ionic strength of one it shoots up.
Sketch and explain the Standard hydrogen electrode
Hydrogen electrode was tested as the arbitrary zero and all electrodes were tested against the hydrogen-Pt electrode.
The tendency for reduction process to occur when all species are at 1M, all gases area at 1bar and an inert metallic electrode is used where no metallic substance is indicated.
Hydrogen gas is in a glass tube containing Pt wire that leads to the Pt electrode within an aqueous solution of H+