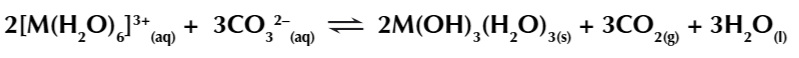4. Na2CO3 hydrolysis of metal aqua ions
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
1
New cards
Metal 2+ ions react w sodium carbonate to form insoluble metal carbonates
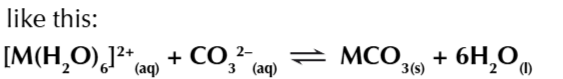
2
New cards
metal 3+ ions dont form M2(CO3)3 species when reacted
3
New cards
metal 3+ ions are stronger acids
so they always form hydroxide precipitates instead
4
New cards
the carbonate ions react w H3O+ ions removing them from the solution and forming bubbles of carbon dioxide gas