Matter, Atomic Structure, Moles
1/54
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
55 Terms
element
the simplest form of matter than cannot be broken down any further by physical nor chemical means
Elements are identified based on their number of ______
the number of protons each of their atoms have
Compound
substance formed when two or more elements are chemically bonded together in a fixed ratio
mixture
two or more, elements, compounds or both combined physically but not chemically bonded
True or false: compounds & elements are pure substances?
true
common techniques of physically separation include
filtration
recrystallisation
evaporation
distillation
paper chromatography
homogenous mixtures
uniform composition
particles are evenly distributed — composition is consistent throughout
heterogenous mixtures
non-uniform composition
particles are not evenly distributed
when to use filtration
separate insolube solids from liquids
when to use recrystallisation
when purifying a solid substance
how does recrystallisation work
relies on difference in solubility
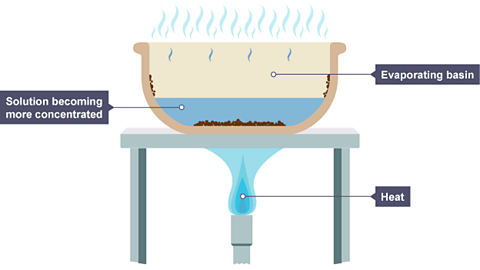
purpose of evaporation
removes a liquid from a solution by heating it (leaving a dissolved solid)
examples of “stubborn” heterogenous mixtures
smoke
milk
tea + milk
pepsi
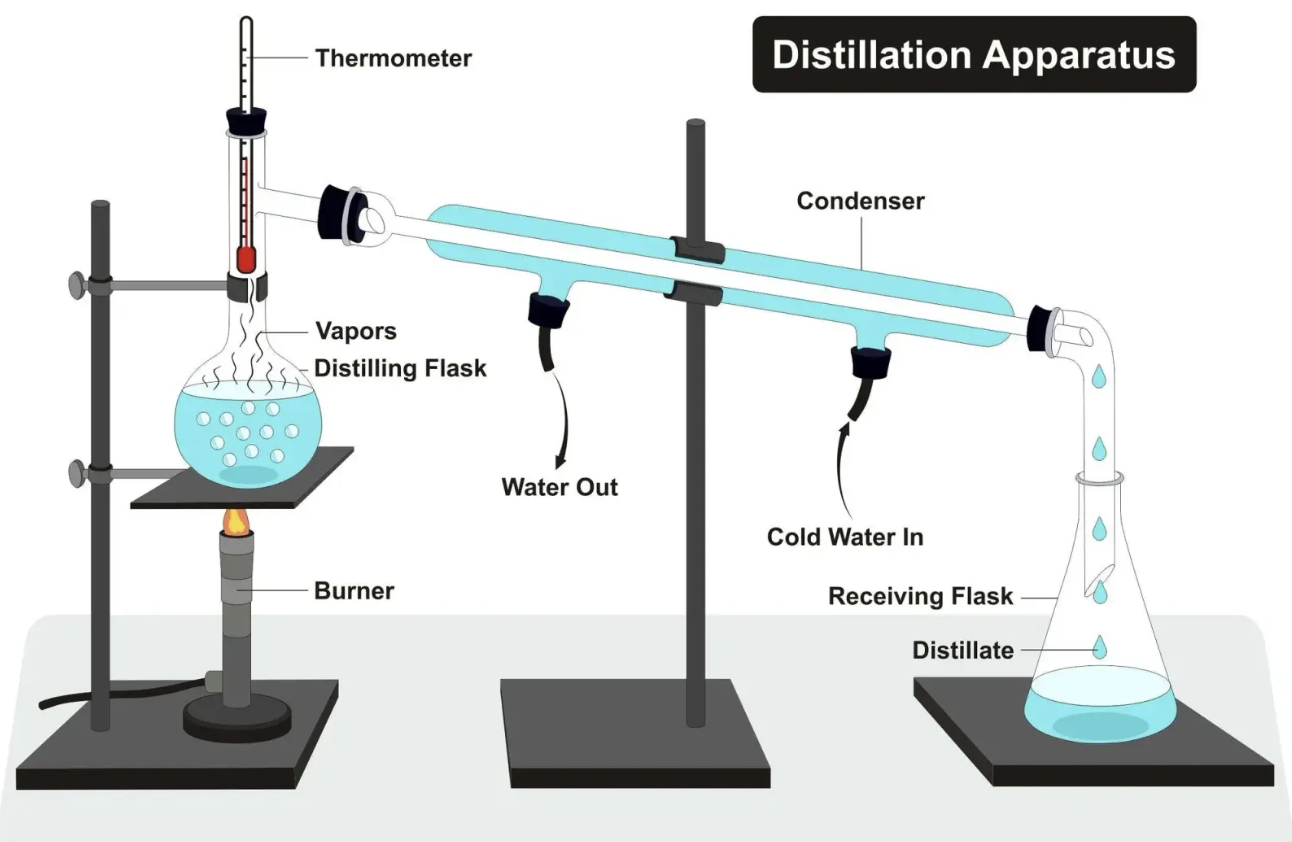
purpose of distillation
separates two or more liquids with different boiling points
ex. separating ethanol from water
how does distillation work
the liquid with the lower boiling point evaporates first, is condensed (cooled) then collected
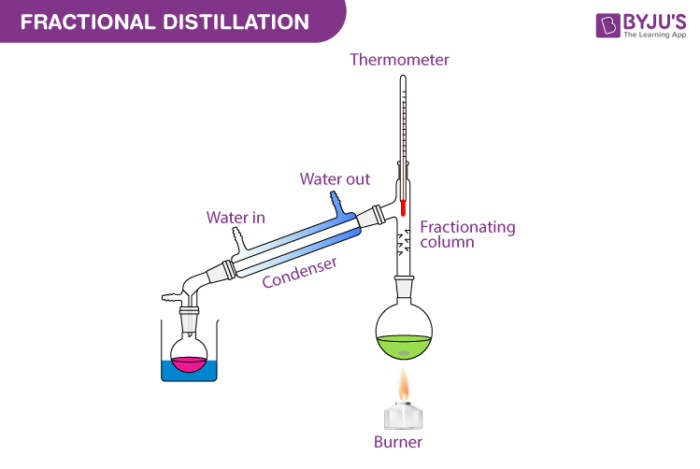
when to use fractional distillation
when aiming to physically separate substances of similar boiling points
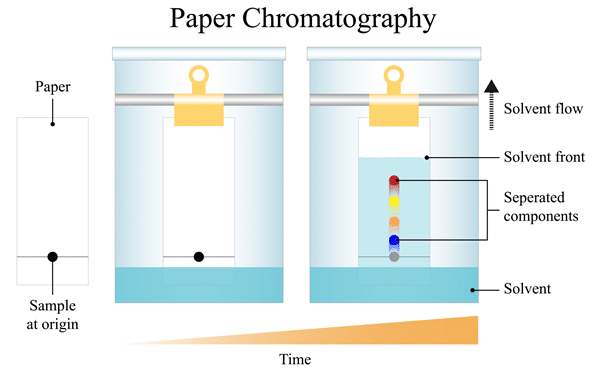
purpose of paper chromatography
separates a mixture based on differences in solubility & polarity
how does paper chromatography work
substances of a particular polarity will dissolve better in a substance of the same polarity (non-polar solute in non-polar solvent, and vice-versa)
the solutes with higher affinity to water will travel further up the page
the solute with higher affinity to paper will travel less distrance
subtances with smaller molecules move faster across the paper
in paper chromatography the paper is referred to as…
stationary phase
in paper chromatography the water is referred to as…
the mobile phase
nucleons
the particles found within the nucleus of an atom (protons and neutrons)
nucleus
dense positively charged core that contains nucleons
purpose of neutrons
offset the repulsion between protons —stabilising the nucleus
isotopes
atoms of the same elements that contain a different number of neutrons
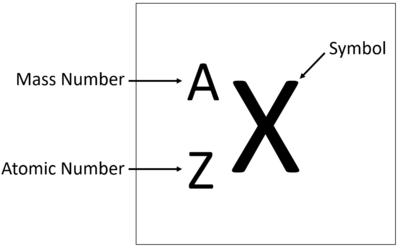
what do the letters A & Z represent
Z = atomic number (number of protons)
A = mass number (# of protons + # of neutrons)
Where are electrons found
Very likely to be in orbitals (likely within the electron cloud)
What is chemical behaviour based on?
number of electrons at atom has
relative atomic mass (Aᵣ)
the weighted average of the masses of an elements isotopes with respect to their to natural abundances
formula for relative atomic mass (Aᵣ)
Aᵣ = ((mass of isotope x abundance) + (mass of other isotope x abundance))/100
how to calculate abundance of isotopes
let the abundance of one isotope be x and the other be 100-x (or 1-x)
then use algebra to find values (solve for x)
what are possible differences between isotopes (and other isotopes)
(natural) abundance
mass
physical properties (boiling point, melting point, solubility, mass)
what does the formula E = h x f represent
the energy of a photon
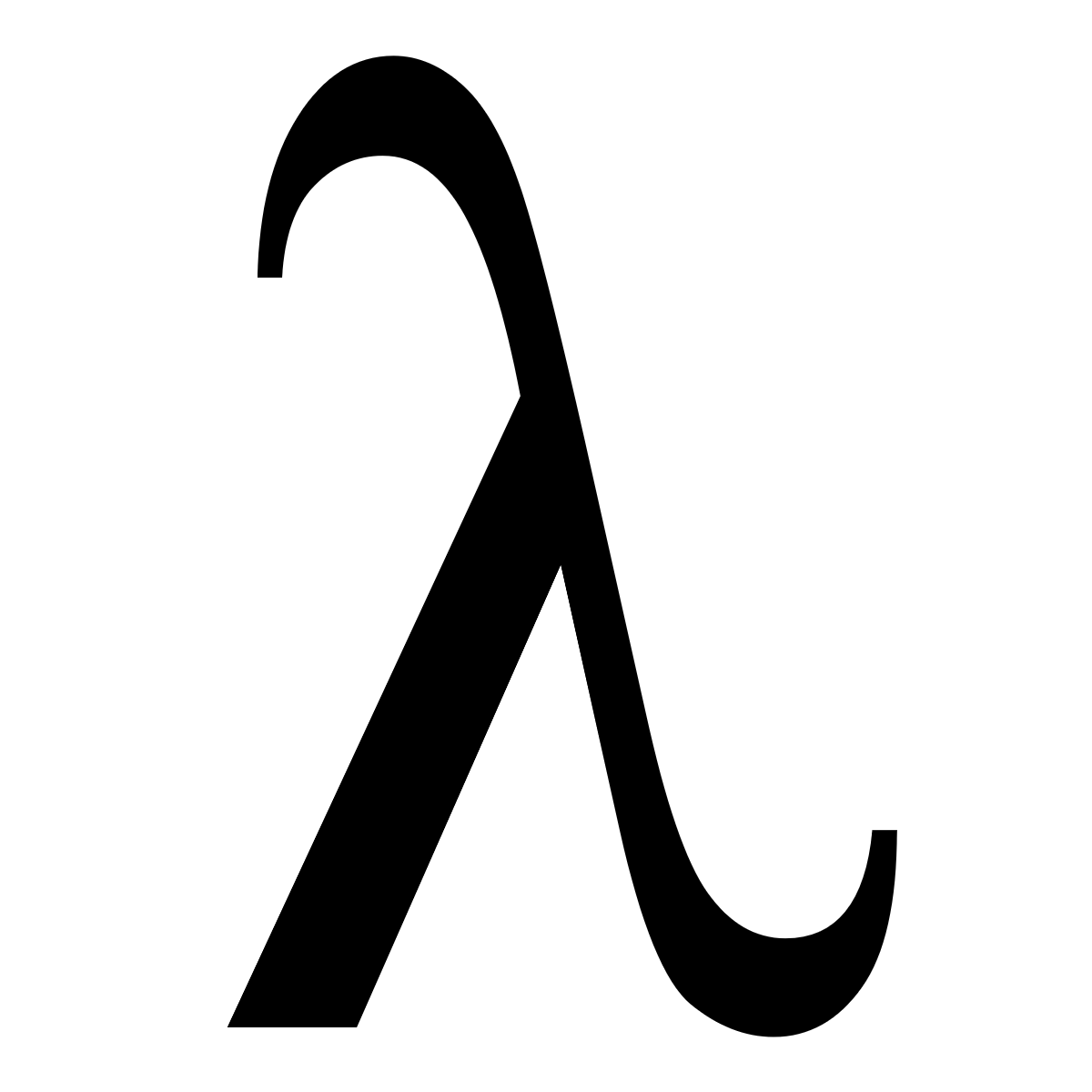
what does this symbol represent
wavelength
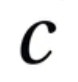
what does this symbol represent
speed of light
how/why do line emission spectra appear
they arise from the transitioning of electrons between specific energy levels
emission spectrum
bright lines corresponding to specific wavelengths of light emitted by an atom
absorption spectrum
dark lines against a continuous background
the dark lines correspond to wavelengths of light absorbed by at atom when an electron excites moving to a higher energy level
emission spectrum
bright coloured lines against a dark background
the colours correspond to a particular wavelength of light
when/how are line emission spectrum produced
when a pure gaseous element is subjected to high voltage under reduced pressure —it emits light
the light is then passed thru a prism which produces the line emission spectrum
why are line emission spectrums different per element
because the electrons of each element’s atoms can only occupy certain 9specific/discrete) energy levels
what form of energy do electrons release when jumping descending from higher energy level to lower
photon
what is the strcuture of hydrogen line emission spectrum
the lines converge at higher energy levels
describe the wavelength of light of an electron transitioning from a higher energy level to n = 1, n = 2 and n = 3
higher energy level → n = 1 = uv region
higher energy level → n = 2 = visible light region
higher energy level → n = 3 = infrared region
what is the formula to calculate the (total) maximum number of electrons in an energy level
2n²
n is the principal quantum number (number of energy levels there are)
Hund’s rule
electrons will fill orbitals singly (all with the same spin) before pairing
Pauli’s exclusion principle
two electrons (an electron pair) sharing an orbital will have opposite spins
where are noble gases located on the periodic table
group 18 (helium, neon, argon, krypton etc)
describe the position of electrons in ions
cations lose electrons from the highest energy level (outermost shell)
anions gain electrons into the highest energy level (outermost shell)
full electron copper (Cu)
1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰
full electron configuration of chromium (Cr)
1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d⁵
why are copper & chromium exceptions for electron configurations
their half/fully-filled d-sublevels are more stable due to reduced electron repulsion (thus less likely to lose electrons)
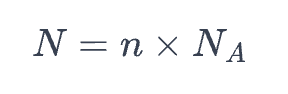
N = number of entity (whether that be atoms, molecules, ions etc)
n = amount of substance in moles
Nₐ = avogadro’s constant
how to calculate the molar mass of a compound
add up the atomic mass of each atom within the compound
(each atom’s atomic mass is multiplied by the number of atoms of it there are present in the compound)
ex. to find the molar mass of CO₂ = molar mass of Carbon + (molar mass of Oxygen x 2) fetched from the periodic table
calculating relative formula mass (Mᵣ)
relative atomic mass of each atom in chemical formula x number of atoms of that element present
add the products together
what unit does molar mass use
g mol ⁻¹ (“grams per mol”)