Unit 9 AP Chemistry ~ Thermodynamics and Electrochemistry
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
46 Terms
Increase in entropy
(5 Bulletpoints)
(Energy and matter more or less dispersed? More or less volume? Temperature increasing or decreasing? More total # of moles in products or reactants?)
Matter more dispersed
Energy more dispersed
More volume
Temperature increases
Total # of moles in the product > total # of moles in the reactants
Calculating absolute entropy change
(What must be consider?)
The # of moles from each substance MUST be considered when calculating entropy

Gibbs free energy change
Describes whether a reaction is thermodynamically favorable and unfavorable
What does it mean when a reaction is thermodynamically favorable?
Proceeds to equilibrium WITHOUT external intervention
∆G°
Gibbs free energy for a chemical process
Finding STANDARD gibbs free energy equation. When is it thermodynamically favorable? What can we neglect out of the equation?
Thermodynamically favorable~ ∆G° < 0
Neglect solids

Kinetic control
Some reactions are thermodynamically favorable reactions but DO NOT make products at a measurable rate
Do reactions under kinetic control have a small or large activation energy?
Large activation energy
What can kinetic control reactions use to speed up the reaction? Does this have an effect on the favorability of the reaction?
Enzymes
Does not have a effect on the favorability of a reaction
What do thermodynamically favorable (∆G° < 0) reactions favor at equilibrium?
They favorable the products at equilibrium. (K > 1)

Connections between K and ∆G° can be made qualitatively through estimation:
What happens when ∆G° is negative?
What happens when ∆G° is positive?
What happens when ∆G° is 0?
Negative: K < 1, reaction favors the products
Positive: K > 1, reaction favors reactants
Zero: Equilibrium
Why are some salts partially soluble and some salts fully soluble?
∆G° indicates if a dissolving process is thermodynamically favorable
What contributes to the value of ∆G°?
∆S° and ∆H° contribute to the value of ∆G°
What would happen to the solubility of a salt when ∆G° is positive?
Slightly soluble
What would happen to the solubility of a salt when ∆G° is negative?
Soluble
What happens when we break attractions? (∆H°)
Negative ∆H°
What happens when we form attractions? (∆H°)
Positive ∆H°
What happens if the ∆H° of a dissolution is positive?
(Endo or exo? Will enthalpy contribute to favorability?)
Endothermic
Enthalpy DOES NOT contribute to favorability
What happens if the ∆H° of a dissolution is negative?
(Endo or exo? Will enthalpy contribute to favorability?)
Exothermic
Enthalpy DOES contribute to favorability
What happens when we break attractions? (∆S°)
Dispersing particles therefore positive ∆S°
What happens when we form attractions? (∆S°)
Organizing particles, therefore negative ∆S°
What happens if the ∆S° of a dissolution is positive?
Increasing
Entropy change DOES contribute to favorability
What happens if the ∆S° of a dissolution is negative?
Decreasing
Entropy change DOES NOT contribute to favorability
∆G° can be determined or predicted if enthalpy and entropy values are available by using the equation…
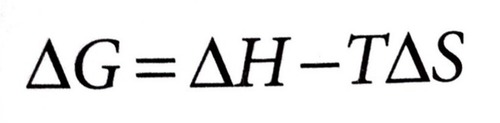
∆G° can also be predicted for enthalpy and entropy signs
(Table)
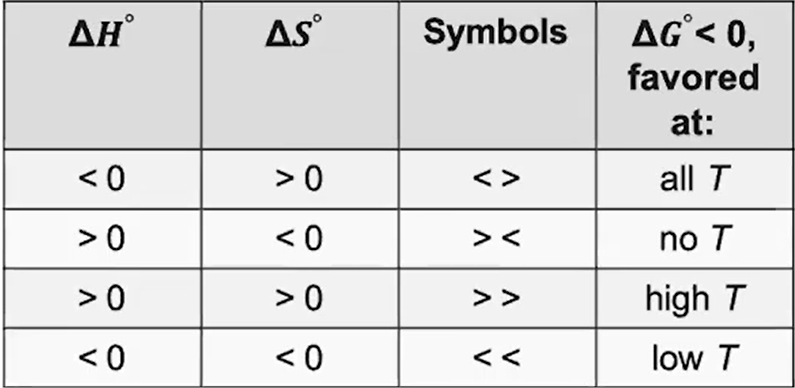
What happens if the ∆G° of a dissolution is positive?
Dissolving is NOT thermodynamically favorable
What happens if the ∆G° of a dissolution is negative?
Dissolving IS thermodynamically favorable
What a unfavorable reaction do to BECOME favorable
Couple with a favorable reaction
If the sum of the ∆G° value is negative then the process is..
Thermodynamically favorable
Galvanic Cell: The Anode Side
(Reduction or oxidation? What happens when there’s a metal reactant? Describe the flow of electrons?)
Oxidation happens here
Metal reactant~ Mass decreases and converted into ions
Flow~ Electrons flow from anode through wire to cathode
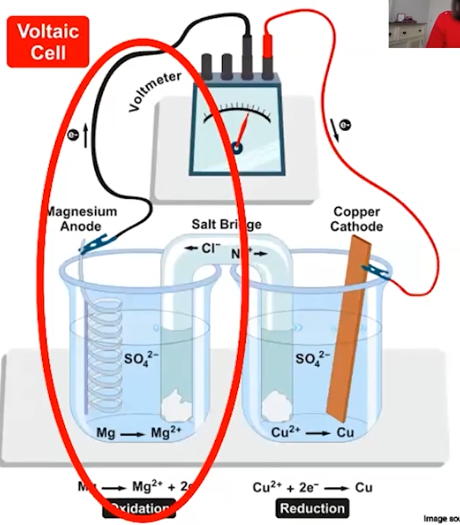
Galvanic Cell: The Cathode Side
(Reduction or oxidation? What happens when there’s a solid? Describe the flow of electrons?)
Reduction
Solid~ Mass of cathode increases
Flow~ Electrons flow into cathode
Galvanic Cell: The Salt Bridge
(What is it? Describe movement in the bridge. What happens if no salt bridge?)
Allows for movement of ions between half-cells
Movement~ Movement of cations in reduction half-cell
Movement of anions in oxidation half-cell
NO SALT BRIDGE NO REACTION
Electrolytic cells
(Thermodynamically favorable or unfavorable? What is needed to power the cell? Single or double chamber? Electron flow? Where do the cations go and what type of reaction is it? Where do the anions go and what type of reaction is it?)
Thermodynamically favorable
Power source needed
Single chamber
Electrons flow~ Anode → Power Source → Cathode
Cations → Cathodes (Reduction)
Anions → Anodes (Oxidations)
What do we use when comparing reduction potential?
(When does it only work? What do the table show?)
Use standard reduction potentials
ONLY work at STP
Tables ONLY list reduction half - reactions
How do we find oxidation half - reaction?
Reverse reduction half - reaction and sign of voltage
What is the standard cell potential equation?

Positive overall cell potential
(Favorable or unfavorable? Which cell?)
Favorable
In voltaic cells
Negative overall cell potential
(Favorable or unfavorable? Which cell?)
Unfavorable
In electrolytic cells
What does cell potential depend on?
Concentrations or pressures of reactants and products
What happens at equilibrium?
(Think magnitude and cell potential)
Magnitude of cell potential decreases, reaching 0 at equilibrium.
Voltaic cell (K > 1): What happens when Q is increased above one?
System is closer to equilibrium and cell potential decreases
EX: Decreasing product concentration
Voltaic cell (K > 1): What happens when Q is decreased below one?
System is further from equilibrium and cell potential increases
EX: Increasing product concentration
Electrolytic cell (K < 1): What happens when Q is increased above one?
System is further from equilibrium and cell potential increases
EX: Increasing product concentration
Electrolytic cell (K < 1): What happens when Q is decreased below one?
(Provide example)
System is closer to equilibrium and cell potential decreases
EX: Decreasing product concentration
Charge flow in electrolysis equation
(What is q)
l = q / t
q~ related to changes of reactants and products in cell
Tips for solving charge from products
(Current, time, faraday’s constant, moles of electrons)
Reminder units:
-Current: Amperes (A), equivalent to coulombs / seconds (C/S)
-Time: needs to be in seconds when using Faradays law equation
-Faraday’s constant: charge per mol of electrons
-Moles of electrons: determined from chemical equation
Use dimensional analysis