QUIZ 2 REDO
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
What does it mean when an element has +1 or -1 like k+1 (when they’re positive or negative) and what does neutral mean?
The + or - means the element has become an ion. Neutral means it’s the same. When this happens you do this to the electron configuration:
Positive (Be+1) = 1s² 2s^1 —> You subtract 1
Neutral (Be) = 1s² 2s²
Negative (Be) = 1s² 2s³
What do you do when an element has positive electrons.
You subtract electrons from the electron configuration
(1s² 2s³ —> 1s² 2s²)
What do you do when an element has negative electrons.
You add electrons to the electron configuration
(1s² 2s³ —> 1s² 2s^4) (3 to 4)
Negative electrons = ____, positive = ________
add, subtract
What is the noble gas shortcut and examples
It is a shortcut for electron configuration. Ex: [Ne] 3s^2
How do you draw the electron cloud structure?
Use the electron configuration for that element.
Example of finding electron cloud structure:
(S): 1s² 2s² 2p^6 3s² 3p^6 —> 2 electrons at row 1, 8 at row 2 and 8 at row 3
2 = row 1
8 = row 2
8 = row 3
How would you draw the electron cloud structure here?
1s² 2s² 2p⁶ 3s² 3p⁶ 4s²
row 1 = 2 electrons
row 2 = 8 electrons
row 3 = 8 electrons
row 4 = 2 electrons
How do you find the highest occupied energy level in an element?
You find the electron configuration and look at the highest row # or look at periodic table and see the row #.
Highest occupied energy level for Iron (Fe)
4
Formula for wave speed
v=λ⋅f
v = speed of the wave (m/s)
λ = wavelength (meters)
f = frequency (Hz, s⁻¹)
what is v is wave speed formula
speed of the waves (m/s)
what is λ in wave speed formula
wavelength (meters)
what is f in wave speed formula
frequency (hz)
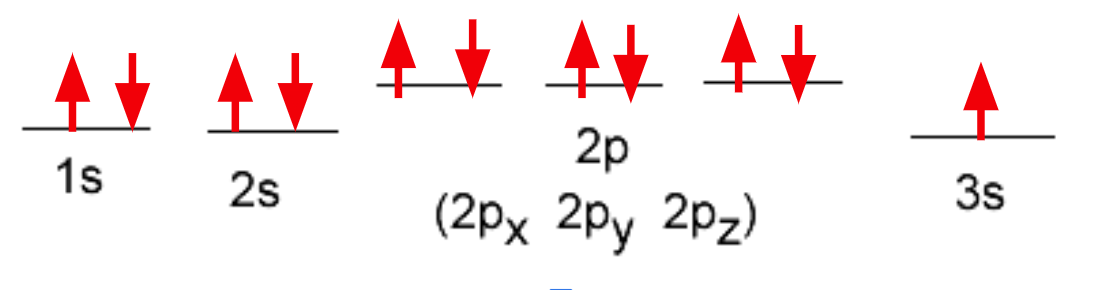
What do the arrows here represent
The number of electrons 1 arrow = 1 electron
Describe the process electrons went under that allowed us to see the light and pattern in the Flame Test and Spectra Lab.
Electrons are naturally in a ground state, meaning they occupy the lowest levels. When energy is acted on the electrons, they absorb it and are in an excited state where they occupy higher energy levels. In this state, electrons are unstable and quickly release that energy to fall back to their ground state. The energy is what we see in the form of light.