Chapter 3
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
50 Terms
In a single molecule of water, two hydrogen atoms are bonded to a single oxygen atom by _____.
polar covalent bonds.
The partial negative charge at one end of a water molecule is attracted to the partial positive charge of another water molecule. What is this attraction called?
a hydrogen bond.
The partial negative charge in a molecule of water occurs because _____.
the electrons shared between the oxygen and hydrogen atoms spend more time around the oxygen atom nucleus than around the hydrogen atom nucleus.
Sulfur is in the same column of the periodic table as oxygen, but has electronegativity similar to carbon. Compared to water molecules, molecules of H2S will _____.
not form hydrogen bonds with each other.
Water molecules can form hydrogen bonds with _____.
compounds that have polar covalent bonds.
Which of the following is a property of liquid water? Liquid water _____.
has a heat of vaporization that is higher than that for most other substances.
Which of the following can be attributed to water's high specific heat?
A lake heats up more slowly than the air around it.
Sugar dissolves in hot tea faster than in iced tea.
The ocean near Portland moderates the temperature.
To act as an effective coolant in a car's radiator, a substance has to have the capacity to absorb a great deal of heat. You have a reference book with tables listing the physical properties of many liquids. In choosing a coolant for your car, which table would you check first?
specific heat.
Water has many exceptional and useful properties. Which is the rarest property among compounds?
Solid water is less dense than liquid water.
Which of the following effects can occur because of the high surface tension of water?
A raft spider can walk across the surface of a small pond.
Which of the following takes place as an ice cube cools a drink?
Kinetic energy in the liquid water decreases.
A dietary Calorie equals 1 kilocalorie. Which of the following statements correctly defines 1 kilocalorie? One kilocalorie equals _____.
1000 calories, or the amount of heat required to raise the temperature of 1 kg of water by 1°C.
Which type of bond must be broken for water to vaporize?
hydrogen bonds.
Why does ice float in liquid water?
Stable hydrogen bonds keep water molecules of ice farther apart than water molecules of liquid water.
Hydrophobic substances such as vegetable oil are _____.
nonpolar substances that repel water molecules.
One mole (mol) of glucose (molecular mass = 180 daltons) is _____.
180 grams of glucose.
When an ionic compound such as sodium chloride (NaCl) is placed in water, the component
atoms of the NaCl crystal dissociate into individual sodium ions (Na+) and chloride ions (Cl-). In contrast, the atoms of covalently bonded molecules (e.g., glucose, sucrose, glycerol) do not generally dissociate when placed in aqueous solution. Which of the following solutions would be expected to contain the greatest number of solute particles (molecules or ions)?
1 liter of 1.0 M NaCl.
The molar mass of glucose is 180 grams per mole (g/mol). Which of the following procedures should you carry out to make a 1 M solution of glucose? Into 0.8 liter (L) of water, dissolve _____.
180 g of glucose and then add more water until the total volume of the solution is 1 L.
You have a freshly prepared 0.1 M glucose solution. Each liter of this solution contains how many glucose molecules?
6.02 × 10^22.
Based on your knowledge of the polarity of water molecules, the solute molecule depicted here is most likely _____.
positively charged.
One mole of the compound above would weigh how many grams? (Note: The atomic masses, in daltons, are approximately 12 for carbon, 1 for hydrogen, and 16 for oxygen.)
60.
How many grams of the compound in the figure above are required to make 1 liter of a 0.5 M solution? (Note: The atomic masses, in daltons, are approximately 12 for carbon, 1 for hydrogen, and 16 for oxygen.)
30.
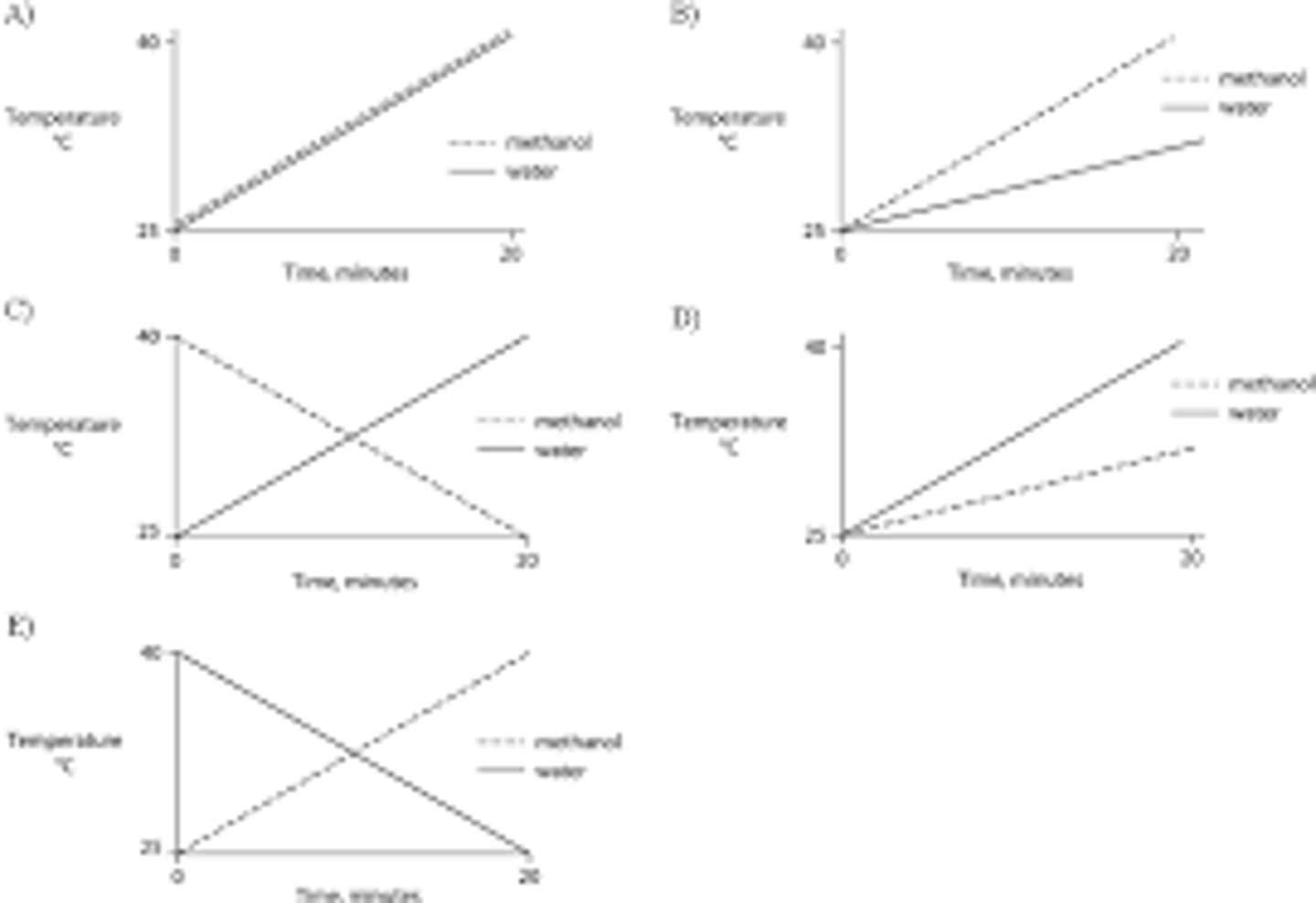
Identical heat lamps are arranged to shine on two identical containers, one containing water and one methanol (wood alcohol), so that each liquid absorbs the same amount of energy minute by minute. The covalent bonds of methanol molecules are nonpolar, so there are no hydrogen bonds among methanol molecules. Which of the following graphs correctly describes what will happen to the temperature of the water and the methanol?
B.
You have two beakers. One contains pure water, the other contains pure methanol (wood alcohol). The covalent bonds of methanol molecules are nonpolar, so there are no hydrogen bonds among methanol molecules. You pour crystals of table salt (NaCl) into each beaker. Predict what will happen.
NaCl crystals will dissolve readily in water but will not dissolve in methanol.
Rank, from low to high, the pH of blood, stomach acid, and urine.
) stomach acid, urine, blood.
A solution with a pH of 5 has how many more protons in it than a solution with a pH of 7?
100 times.
Consider the following reaction at equilibrium: CO2+H2O->H2CO3. What would be the effect of adding additional H2CO3?
It would drive the equilibrium dynamics to the left.
A strong acid like HCl _____.
dissociates completely in an aqueous solution.
Which of the following dissociates completely in solution and is considered to be a strong base (alkali)?
NaOH
A 0.01 M solution of a substance has a pH of 2. What can you conclude about this substance?
It is a strong acid that dissociates completely in water.
A solution contains 0.0000001 (10-7) moles of hydroxyl ions [OH-] per liter. Which of the following best describes this solution?
neutral.
What is the pH of a solution with a hydroxyl ion (OH-) concentration of 10-12 M?
pH 2.
Which of the following solutions would require the addition of the greatest amount of base to bring the solution to neutral pH?
gastric juice at pH 2
What is the hydrogen ion (H+) concentration of a solution of pH 8?
10^-8 M.
If the pH of a solution is decreased from 9 to 8, it means that the concentration of _____.
H+ has increased tenfold (10X) compared to what it was at pH 9.
One liter of a solution of pH 2 has how many more hydrogen ions (H+) than 1 liter of a solution of pH 6?
10,000 times more.
Which of the following statements is true about buffer solutions?
They maintain a relatively constant pH when either acids or bases are added to them.
One of the buffers that contribute to pH stability in human blood is carbonic acid (H2CO3). Carbonic acid is a weak acid that, when placed in an aqueous solution, dissociates into a bicarbonate ion (HCO3-) and a hydrogen ion (H+), as noted below.
If the pH of blood drops, one would expect _____.
the HCO3- to act as a base and remove excess H+ by the formation of H2CO3.
One of the buffers that contribute to pH stability in human blood is carbonic acid (H2CO3). Carbonic acid is a weak acid that, when placed in an aqueous solution, dissociates into a bicarbonate ion (HCO3-) and a hydrogen ion (H+), as noted below.
If the pH of blood increases, one would expect _____.
a decrease in the concentration of H2CO3 and an increase in the concentration of HCO3-.
Assume that acid rain has lowered the pH of a particular lake to pH 4.0. What is the hydroxide ion concentration of this lake?
1 × 10^-10 mol of hydroxide ions per liter of lake water.
Consider two solutions: solution X has a pH of 4; solution Y has a pH of 7. From this information, we can reasonably conclude that _____.
the concentration of hydrogen ions in solution X is 1000 times as great as the concentration of hydrogen ions in solution Y.
A beaker contains 100 milliliters (mL) of NaOH solution at pH = 13. A technician carefully pours into the beaker 10 mL of HCl at pH = 1. Which of the following statements correctly describes the result of this mixing?
The pH of the beaker's contents will decrease.
Increased atmospheric CO2 concentrations might have what effect on seawater?
Seawater will become more acidic, and carbonate concentrations will decrease.
How would acidification of seawater affect marine organisms? Acidification of seawater would _____.
decrease dissolved carbonate concentrations and hinder growth of corals and shell-building animals.
One idea to mitigate the effects of burning fossil fuels on atmospheric CO2 concentrations is to pipe liquid CO2 into the ocean at depths of 2500 feet or greater. At the high pressures at such depths, CO2 is heavier than water. What potential effects might result from implementing such a scheme?
increased acidity and decreased carbonate concentrations in the deep waters.
If the cytoplasm of a cell is at pH 7, and the mitochondrial matrix is at pH 8, then the
concentration of H+ ions _____.
is 10 times higher in the cytoplasm than in the mitochondrial matrix.
Carbon dioxide (CO2) is readily soluble in water, according to the equation CO2 + H2O ↔ H2CO3. Carbonic acid (H2CO3) is a weak acid. If CO2 is bubbled into a beaker containing pure,
freshly distilled water, which of the following graphs correctly describes the results?
B.
The loss of water from a plant by transpiration cools the leaf. Movement of water in transpiration requires both adhesion to the conducting walls and wood fibers of the plant and cohesion of the molecules to each other. A scientist wanted to increase the rate of transpiration of a crop species to extend its range into warmer climates. The scientist substituted a nonpolar solution with an atomic mass similar to that of water for hydrating the plants. What do you expect the scientist's data will indicate from this experiment?
Transpiration rates will fall to zero as nonpolar compounds do not have the properties necessary for adhesion and cohesion.
In living systems molecules involved in hydrogen bonding almost always contain either oxygen or nitrogen or both. How do you explain this phenomenon?
Oxygen and nitrogen are elements with very high attractions for their electrons.