Lecture 13: Introduction to Biosensors
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
What is a Biosensor?
Biosensors are analytical devices that combine a biological detecting elements with a transducer to produce a signal
Sensor is very specific for analyte
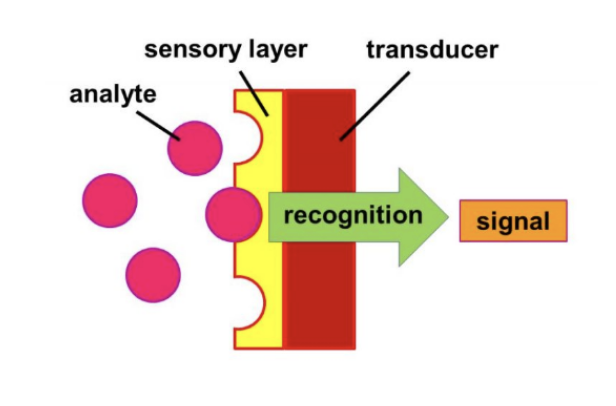
Elements of a Biosensor
Bioelement: Enzyme, Ab, Nucleic acid, tissue, microbial, polysaccharide
sensor element: electric potential/ current/ conductance/ impedance, intesity and phase of EM radiation, mass, temperature, viscosity
Coupling the Biorecognition Element to the Transducer
Membrane Entrapment
A semipermeable membrane separates the analyte and the bioelement
only allows analyte of interest to diffuse through: eg gas
inconsistent signals
Physical Adsorption
Dependent on van der Waals forces, hydrophobic forces, hydrogen bonds, and ionic forces to attach the biomaterial to the surface of the sensor
weak bonds = easy to dislodge biomaterial, flow through system not ideal
Porous Entrapment
Based on forming a porous encapsulation matrix around the biological material which helps bind it to the sensor
more stable, but when carbon dries it can distort the analyte, less sensitive sensor
Covalent Bonding
The sensor surface is treated as a reactive group to which the biological materials can bind
stronger, best
Essential Performance Characteristics of a Biosensor
The biocatalyst must be highly specific for analyte of interest
The sensor should be independent of physical parameters
eg stirring, pH and temperature
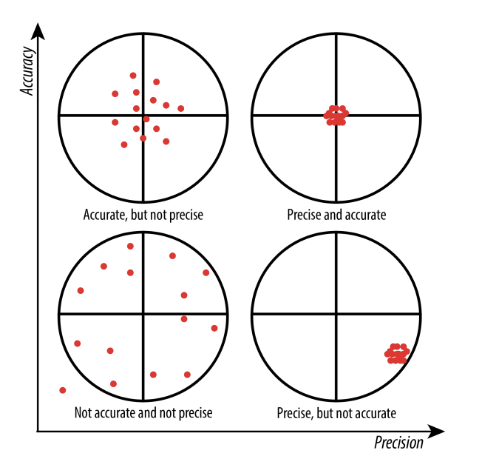
The sensor response should be accurate, precise, reproducible and linear over an appropriate analytical range
The biosensor should be small and biocompatible if it is to be used in vivo
The biosensor should be cheap, portable and capable of being used by semi-skilled operators (eg glucose sensors)
There should be a commercial market for the biosensor
Cholesterol Monitoring (CardioChek)
blood sample is applied to test strip and chemical reaction occurs producing a colour change
reflectance photometrynused to measure the colour reaction and compares the info to the calibration curve stored in the unit.
Cholesterol need to be measured at 6 monthly intervals – So no market
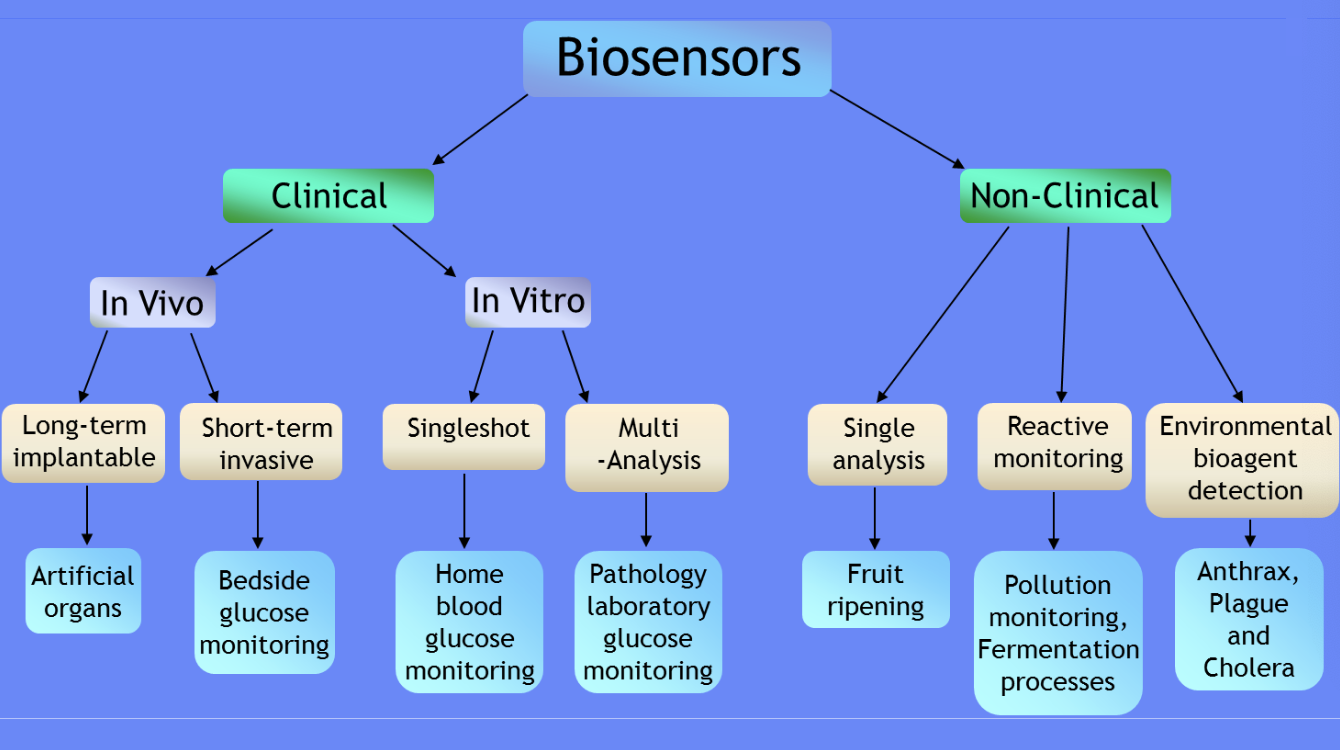
Market for biosensors
Clinical biosensors: Medical applications for patient diagnostics and monitoring
In Vivo (inside the body)
Long-term implantable: Artificial organs
Short-term invasive: Bedside glucose monitoring
In Vitro (outside the body/lab)
Singleshot: Home blood glucose monitoring
Multi-analysis: Pathology laboratory glucose monitoring
Non-clinical biosensors: Applications outside medical settings
Single analysis: Fruit ripening sensors
Reactive monitoring: Pollution monitoring, fermentation processes
Environmental bioagent detection: Detection of pathogens like anthrax, plague, and cholera
The Physical Characterisation of Biosensor Sensing Properties
Optical: direct optical dectection
Physical: thermometric
Electrochemical: conductometric, potentiometric, amperometric
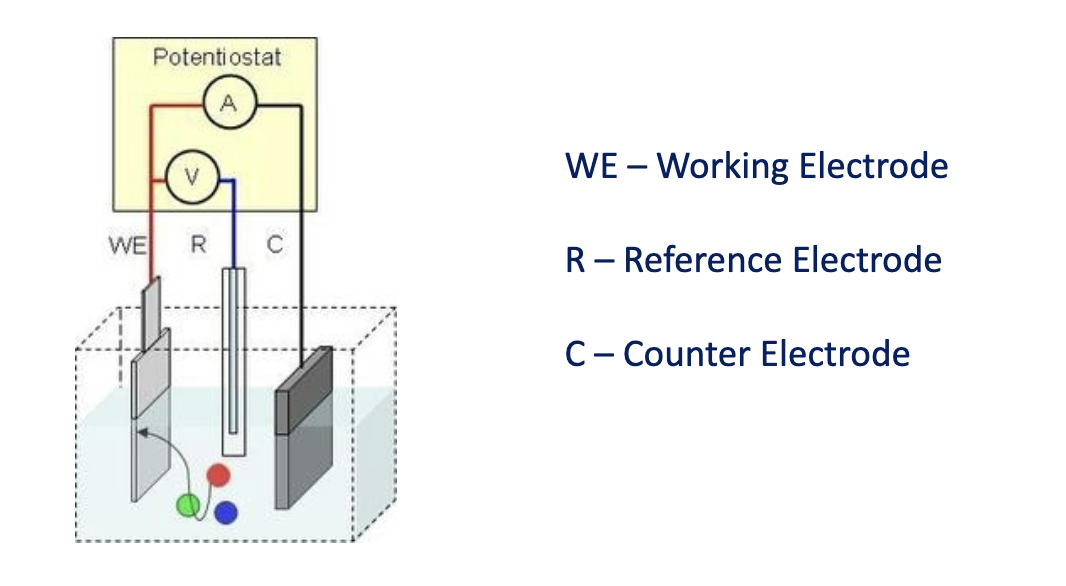
Electrochemical Biosensors
Many chemical reactions produce or consume ions or electrons, causing some change in the electrical properties of the solution that can be used as a measuring parameter
no electrochemical reactions
Conductometric - measures conductance (inverse of resistance)
Electrochemical biosensors with electrochemical reactions.
Potentiometric - measures changes in potential difference (consumption of ions)
Amperometric - measures current (consumption of electrons)
Ohm’s Law
Ohm's Law deals with the relationship between voltage and current.
‘The potential difference (voltage) across an ideal conductor (i.e. no resistance) is proportional to the current through it.’
Ohm's Law: V = I R
V = potential difference between two points
I = current flowing through the resistance.
R = resistance to current flow.
Conductometric Biosensors
Conductometry is a method with no electrochemical reactions for the electrodes to detect.
The most important property of the electrolytic solution is its conductivity
varies with a wide range of biological reactions.
Based on measuring changes in resistance of a selective material.
Inverse of resistivity = conductivity
called conductometric sensors or chemiresistors
There are two types of these sensors:
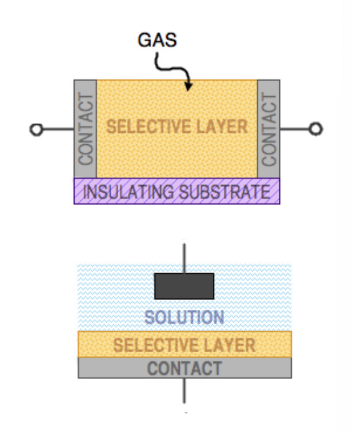
measuring gas: A selective material, which can change its conductivity upon interaction with chemical species is clamped between two contact electrodes and the resistance of the entire device is measured
no gas = no current = maximum resistance
measuring solution: the chemically interactive layer is at the top of an electrode, which is immersed in the solution of electrolyte
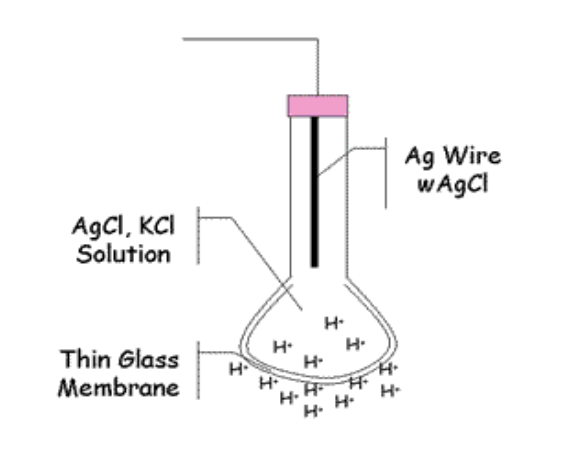
Potentiometric (pH) Biosensors
Potentiometric biosensors use ion-selective electrodes
transduce the biological reaction into an electrical signal.
This consists of an immobilised enzyme membrane surrounding the probe from a pH-meter where the catalysed reaction generates or absorbs hydrogen ions.
The reaction occurring next to the thin sensing glass membrane causes a change in pH
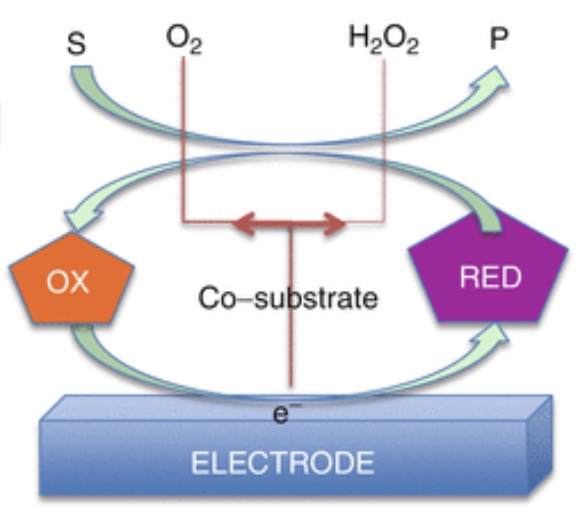
Amperometric Biosensors
Amperometric is a high-sensitivity biosensor that can detect electroactive species present in biological test samples.
Since the biological test samples may not be intrinsically electroactive, enzymes are needed to catalyze the production of reactive species.
In this case, the measured parameter is current.
Commercially Successful Biosensors
Blood Glucose: Diabetes Monitoring
Pregnancy Test: ClearBlue
Blood Glucose Biosensor
85% of biosensors are glucose biosensors (~£2.5billion)
mainly due to the prevalence of diabetes in developed nations
Need for repeated measurement – high demand for consumable electrodes
Diabetes complecations
270/380 million develop complications
70% eye diseases
70% heart diseases
40% nerve damage
30% kidney disease
>60% of all non-traumatic limb amputations are due to diabetes
Routine monitoring of blood glucose is the key to effective management of diabetes
Dexcom
Redox Enzyme Electrochemistry
Central to Amperometric, Biosensor Based Glucose Measurement
most commonly used enzymes in the design of glucose biosensors contain redox groups that change redox state during the biochemical reaction.
Enzymes of this type include glucose oxidase (GOx) and glucose dehydrogenase (GDH)
Redox Electrochemistry
Oxidase enzymes oxidise their substrates > accepts electrons > inactivated reduced state
enzymes return to active oxidised state on electrode
electrons can be transferred to molecular oxygen, resulting in the production of hydrogen peroxide (H2O2):
glucose + O2 → gluconolactone + H2O2
Glucose may also be oxidised by GDH, it relies on NAD+ acting as a cofactor, rather than oxygen as a cosubstrate.
glucose + NAD+ → gluconolactone + NADH
Advantages/Disadvantages of redox electrochemistry
Glucose Oxidase
Advantages: Inexpensive
Disadvantages: Requires oxygen as a cosubstrate. depleted O2 in the sample = decrease performance
Glucose Dehydrogenase
Advantages: Oxygen independent
Disadvantages: The cofactor (NAD+) is expensive and unstable.
Biosensor ‘Generations’
No direct connection between enzyme and electrode. Measures end product of substrate conversion eg H2O2
The use of a mediator (eg ferrocyanide) to link active site to electrode surface
Direct electron transfer between enzyme and electrode surface
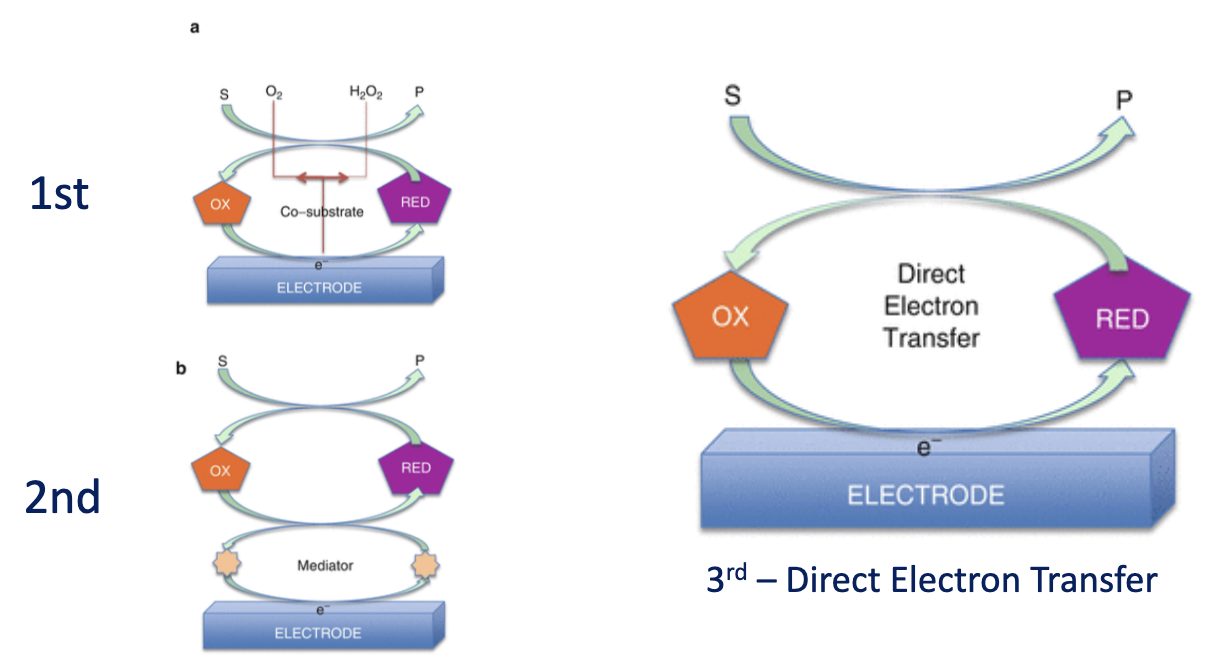
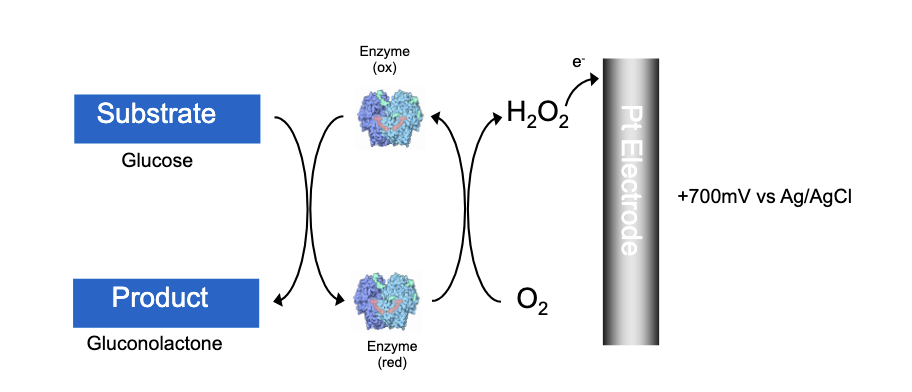
1st Generation Glucose Biosensor: amperometric glucose biosensors
Professor Clark Jr: direct detection of H2O2 oxidation at the electrode surface +ve charge
The earliest approaches to the construction of amperometric glucose biosensors were based on GOx immobilised close to an electrode
The depletion of oxygen was monitored, using a Clark oxygen electrode
disadvantage: high potential of electrode other things can be oxidisese (paracetamol) to form current
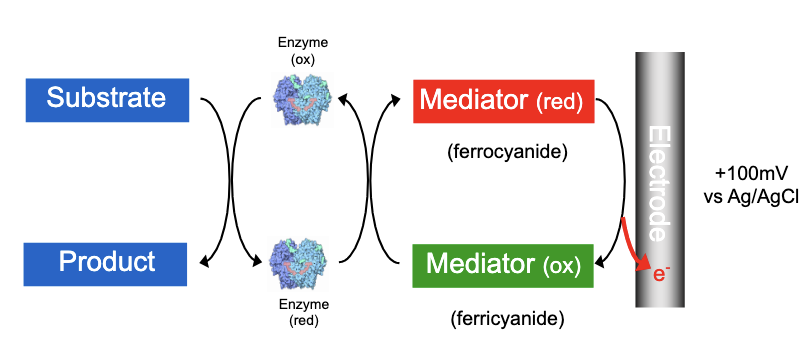
2nd Generation Glucose Biosensor: Redox Mediators
The use of redox mediators facilitated a transfer of electrons in enzyme electrodes which:
was independent of the local oxygen concentration
allowed operation at much lower potentials, minimising detection of interferents (eg ascorbic acid, urate and paracetamol)
These redox couples, or mediators, are able to shuttle electrons between the redox centre of the enzyme and the electrode
most important examples of this class are mediators based on ferrocene and its derivatives
They have a wide range of redox potentials
Their redox potentials are independent of pH
They are easy to manufacture
Disadvantage: mediators are toxic so can’t be used in vivo biosensors
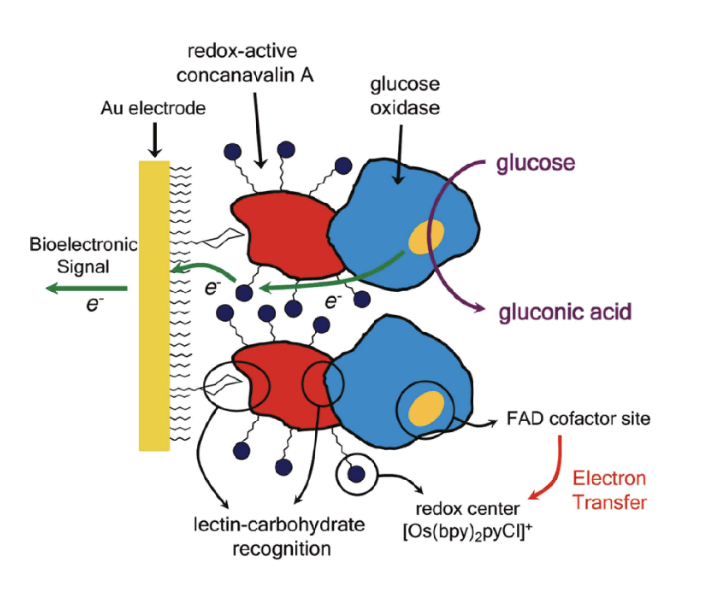
Third Generation Glucose Sensor
Direct electron transport between enzyme active site and the electrode surface
no reagent or mediators required
Redox enzymes can be:
ectrinsic: active site on surface, easy
GOx intrinsic: active site deep within structure
Instead of (toxic) mediators, the electrode can perform direct electron transfers using organic conducting materials based on charge-transfer complexes
The structure of glucose oxidase
Dimeric enzyme.
Co-factor FAD/FADH2 (1 per subunit)
Glucose Oxidase is an intrinsic enzyme with an active site buried deep within the protein structure
Difficult to gain access to the active site for electrochemistry
Access to GOx active site
FAD active site 'buried' in cavity 13Å from surface of protein.
Electrostatic surface at entrance to active site
Positively charged lysine residues used to orientate substrate to AS
potentially use to bind to electrode
Covalent immobilisation of GOx at a modified gold electrode
Positively charged residues of GOx (e.g. lysine), in red.
Suitable for reaction with cross-linkers such as DTSSP (3,3'-dithiobis(sulfosuccinimidyl propionate)
DTSSP = dimer linked by diS bonds + carboxyl groups
DTSSP is a water-soluble crosslinker that contains amine-reactive NHS-ester ends around an 8-atom spacer arm, whose central disulfide bond can bind covalently to gold
Binding of Gox to Gold Electrode using DTTSP
disulphide group bonds covalently to the gold electrode surface
DTSSP dimer split into two monomers
carboxyl groups presented
GOx, have several primary amines in the side chain of lysine that are available as targets for sulfo-NHS- ester crosslinking reagents
Amine groups of Gox bind to carbonyl groups of DTSSP to covalently link the enzyme to the electrode surface
Direct electrochemistry of GOx
glucose + GOx /FAD → gluconolactone + FADH2
GOx /FADH2 →(electrode)→ GOx /FAD + 2H+ + 2e-
Catalytic oxidation current from DTSSP immobilized GOx electrode plotted as function of [glucose]
![<ul><li><p>glucose + GOx /FAD <strong>→ </strong>gluconolactone + FADH<sub>2</sub></p></li><li><p>GOx /FADH<sub>2</sub> <strong>→(electrode)→ </strong>GOx /FAD + 2H<sup>+</sup> + 2e-</p></li><li><p>Catalytic oxidation current from DTSSP immobilized GOx electrode plotted as function of [glucose]</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/ef8ff292-820b-4b67-8898-8af1b5006eed.png)