Chemistry II Lesson 3: Stereochemistry
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
49 Terms
The first step in assigning R and S designations is to prioritize the four groups attached to the chirality center. Arrange the following in terms of increasing priority (periodic table: http://www.sbcs.qmul.ac.uk/iupac/AtWt/table.gif):
I. Fluorine
II. Chlorine
III. Bromine
IV. Hydrogen
(A) IV < II < I < III
(B) IV < I < II < III
(C) III < I < II < IV
(D) III < II < I < IV
(B) IV < I < II < III
In order of increasing priority: Hydrogen (1.01 amu) < Fluorine (18.99 amu) < Chlorine (35.45 amu) < Bromine (79.90 amu)
CRB Based on the previous question, which of the following strictly determines priority of bound elements?
(A) Electronegativity
(B) Atomic Number
(C) Ionic Radius
(D) Number of Neutrons
(B) Atomic Number
The atomic number of an element is used to determine the priority of different bound elements.
CRB True or False? If a molecule has one or more chiral centers, it must be a chiral molecule.
False. A molecule with chirality centers could still have a plane of symmetry, which would make the molecule achiral.
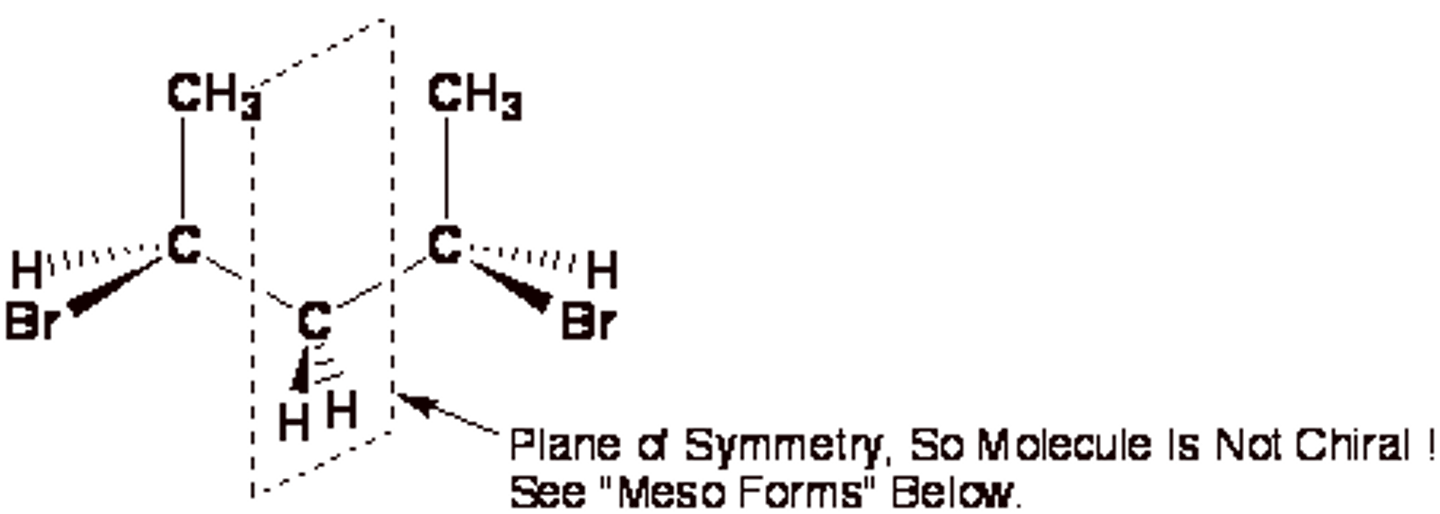
The second step in assigning R and S designations is to arrange what group projecting away?
The lowest priority group.
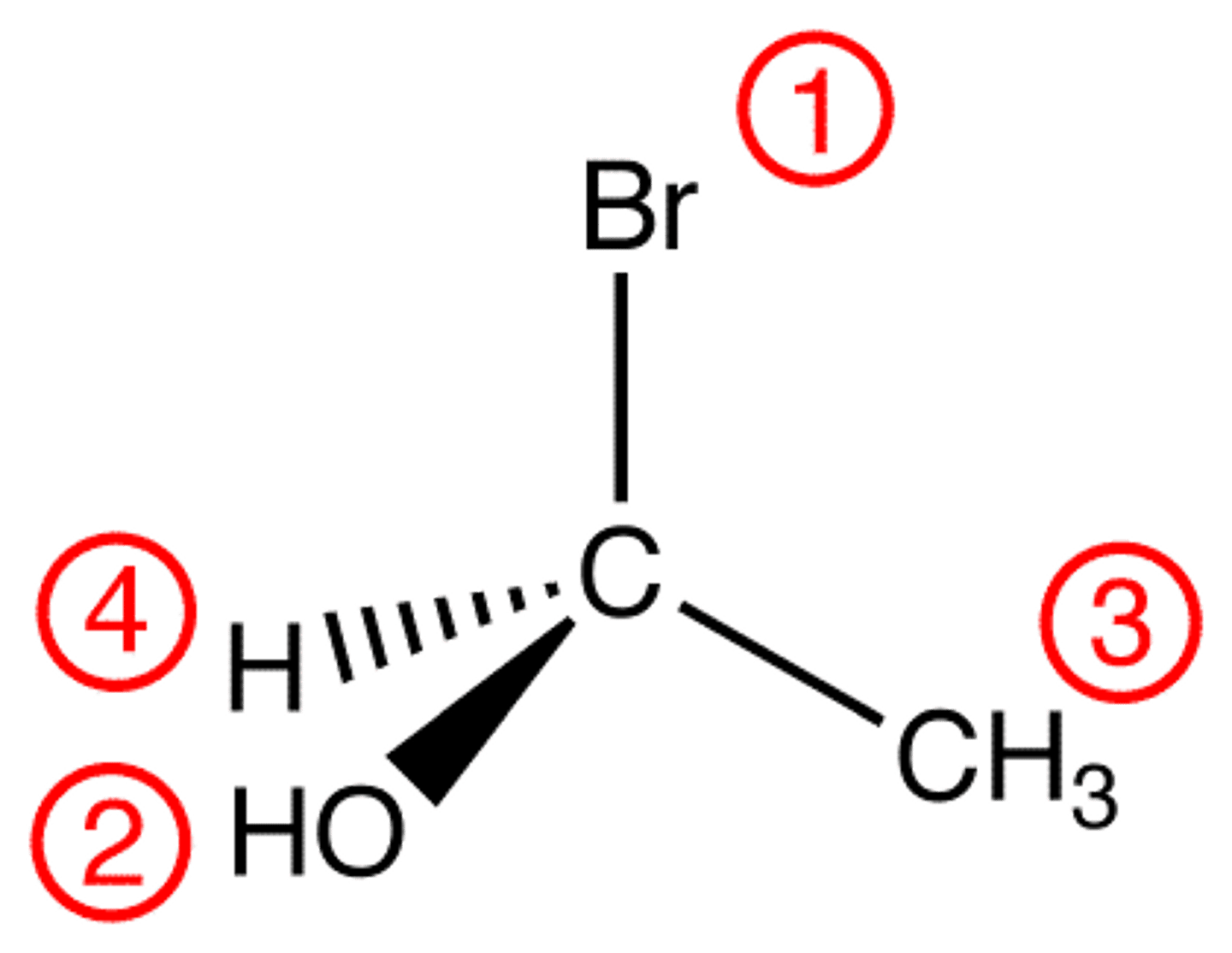
The final step is to determine if the sequence 1-2-3 is clockwise or counterclockwise. If it is clockwise, the designation would be __________. If it is counterclockwise, the designation would be __________.
(A) d, l
(B) R, S
(C) S, R
(D) l, d
(B) R, S
If it is clockwise, the designation would be R. If it is counterclockwise, the designation would be S.
l and d refer to how a chiral molecule rotates a plane of polarized light.
CRB (R)-Carvone and (S)-Carvone are enantiomers. They have which of the following properties in common?
I. Melting Point
II. Boiling Point
III. Density
(A) I Only
(B) I and II Only
(C) III Only
(D) I, II, and III
(D) I, II, and III
(R)-Carvone and (S)-Carvone are enantiomers and as such would have the same melting point, boiling point, and density.
CRB Which of the following physical characteristics could vary between enantiomers?
I. Affinity for enzymes
II. Molecular Weight
III. Direction of polarized light
(A) III only
(B) I and II only
(C) I and III only
(D) I, II and III
(C) I and III only
Enantiomers can polarize light and opposite directions and have different affinities for enzymes (for example, only L-amino acids are used in protein synthesis).
NOTE For the following cards, change your settings so that the Definition side is shown first! This is so you can see the compounds for R and S practice before seeing the answers.
NOTE For the following cards, change your settings so that the Definition side is shown first! This is so you can see the compounds for R and S practice before seeing the answers.
R
Priority of groups:
1. Br
2. Cl
3. CH3
4. H
R or S?
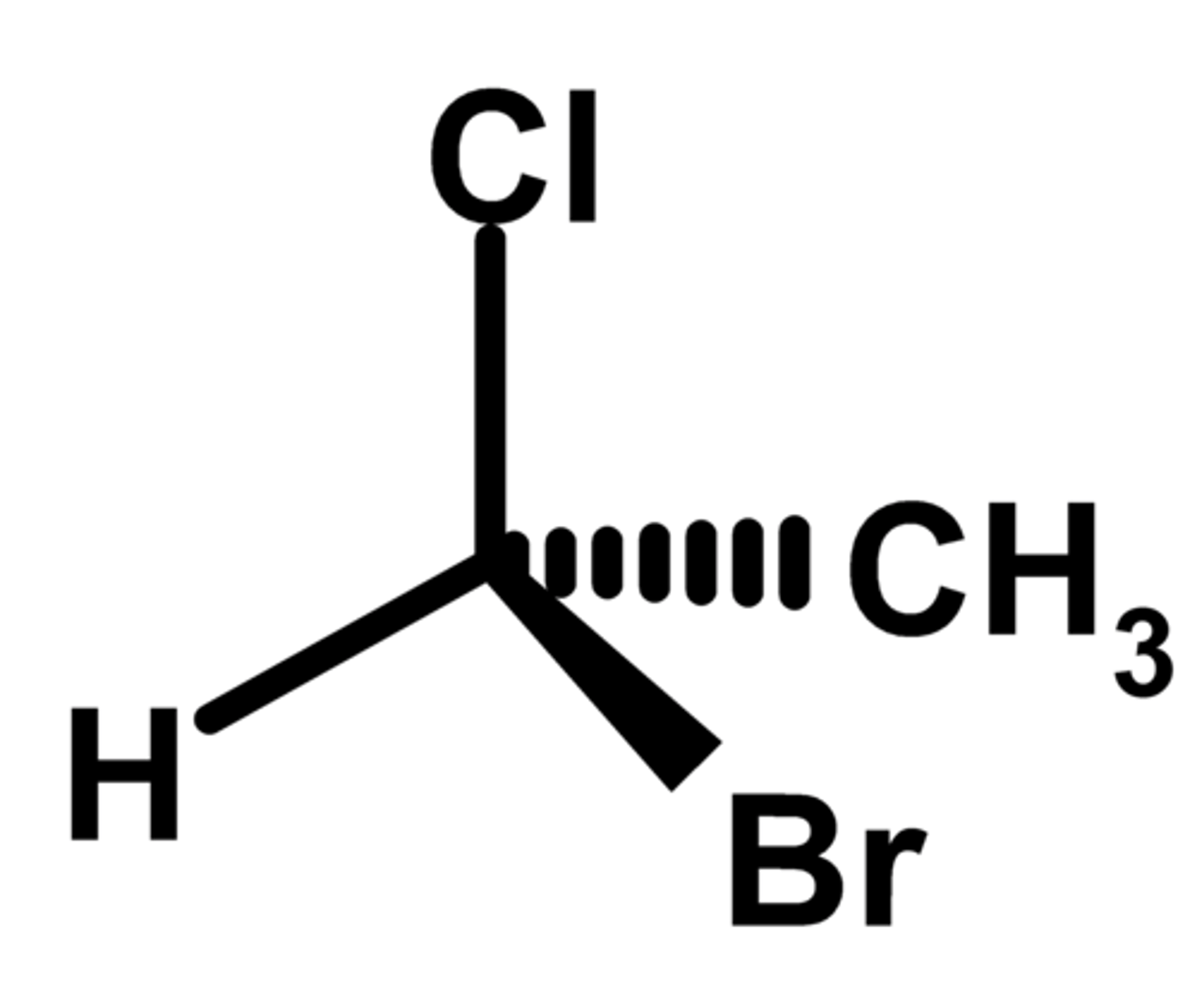
R
Priority of groups:
1. OH
2. COOH
3. CH3
4. H
R or S?
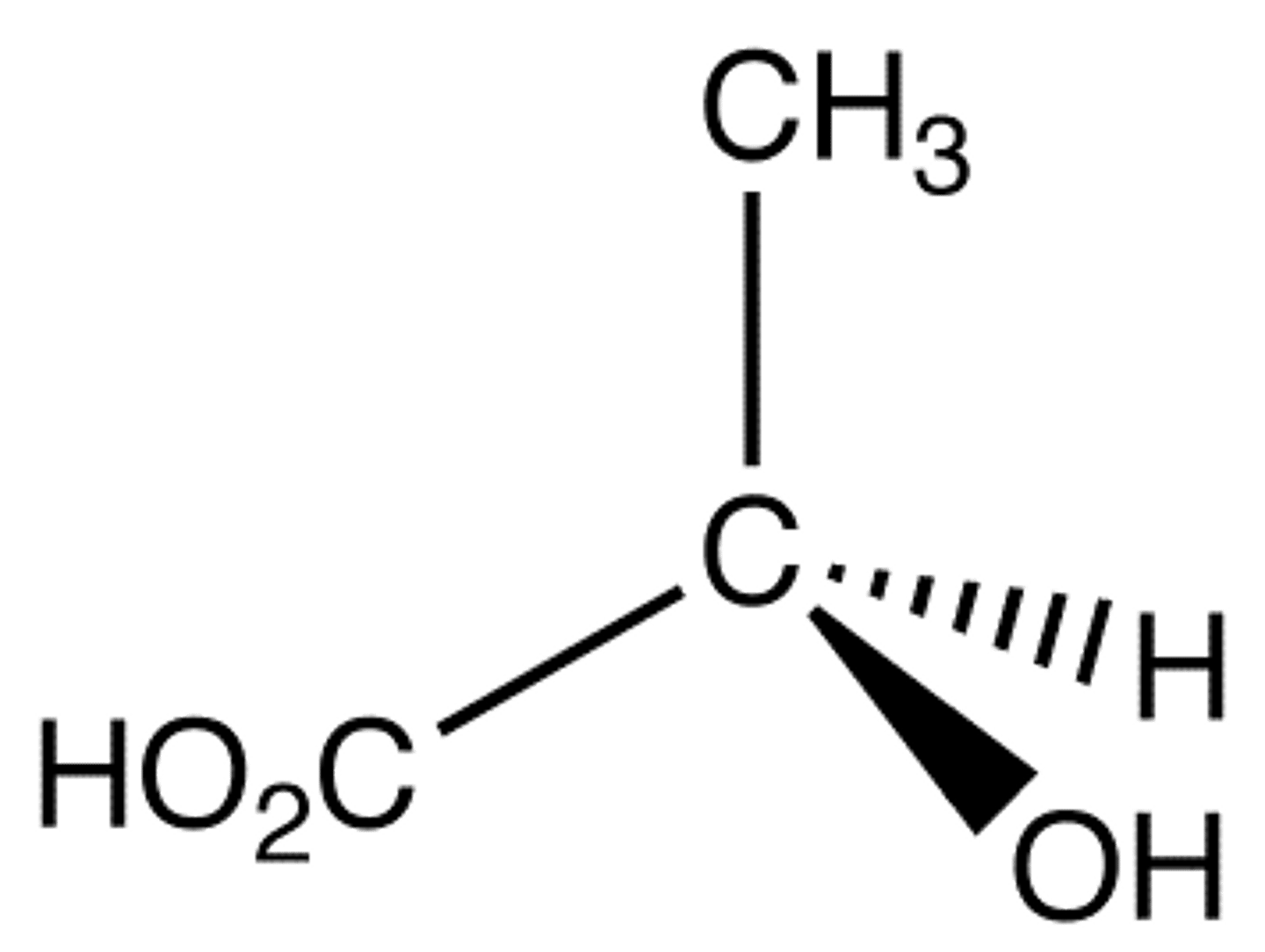
R
Priority of Groups:
1. NH2
2. C=O
3. C - C - Cl
4. H
R or S for the carbon labeled 2?
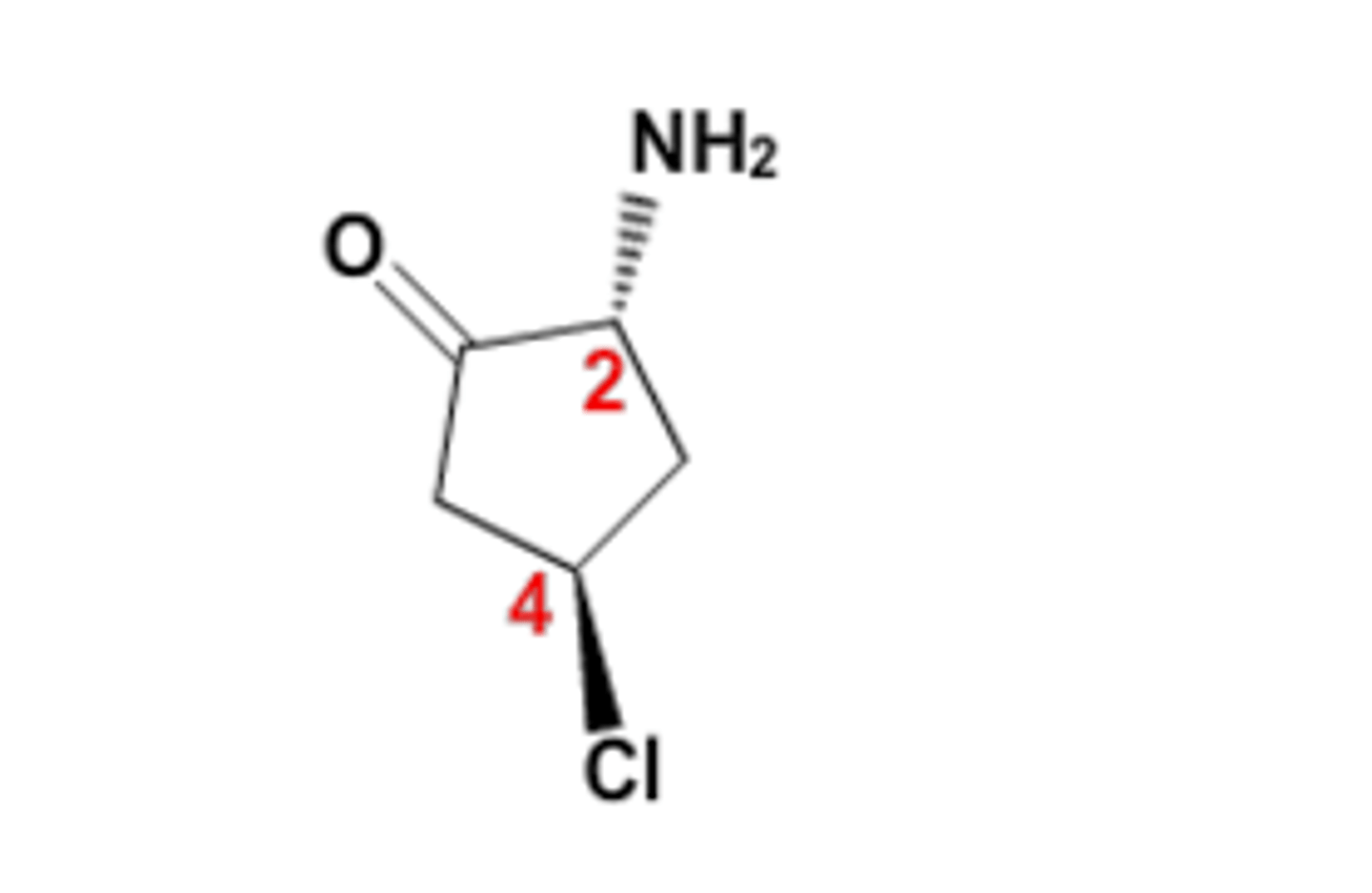
R
1. Cl
2. C - C = O
3. C - C - NH2
4. H
R or S for the carbon labeled 4?
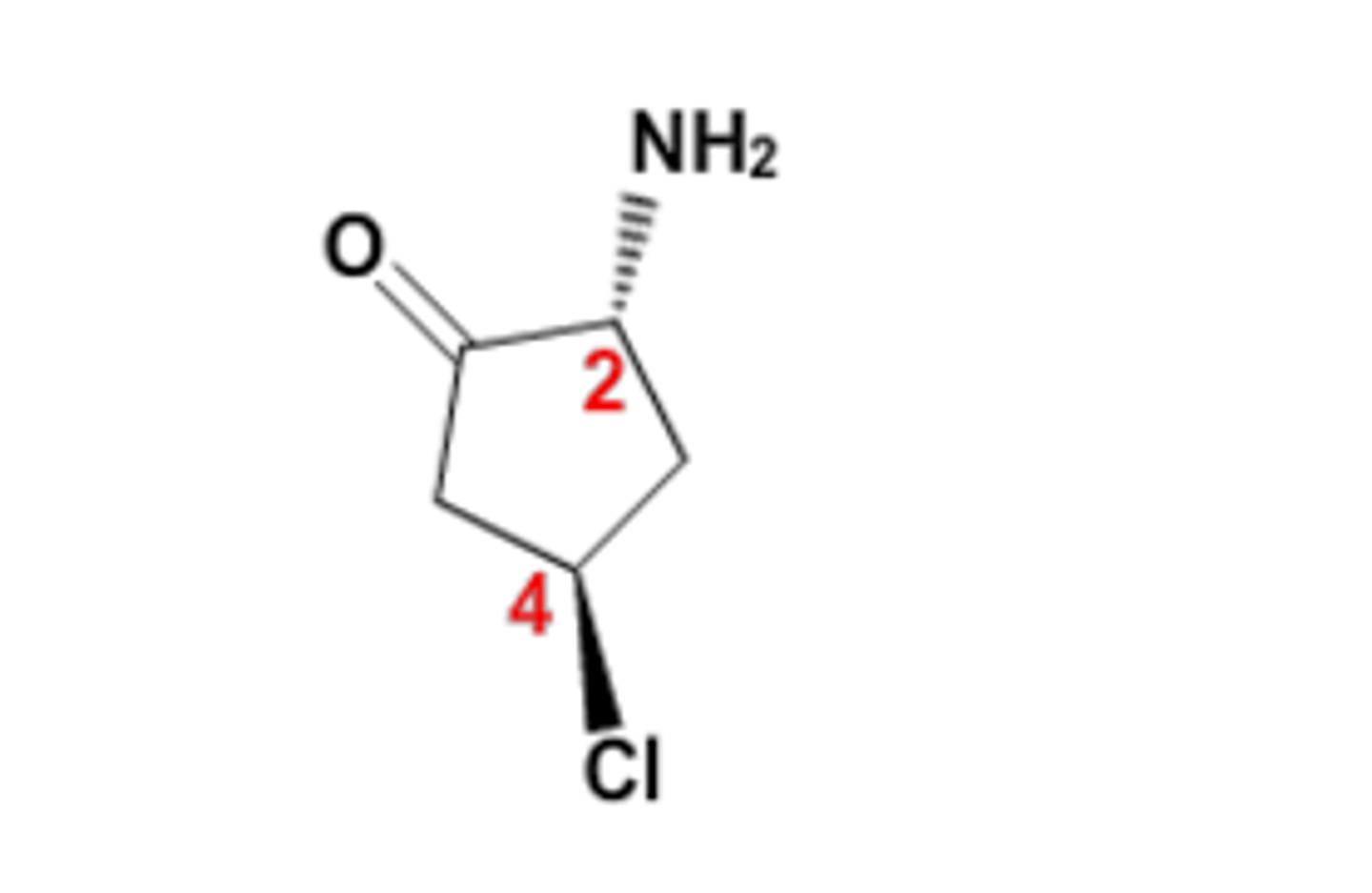
Neither. There are only 3 unique bonds.
R or S for the carbon labeled 1?
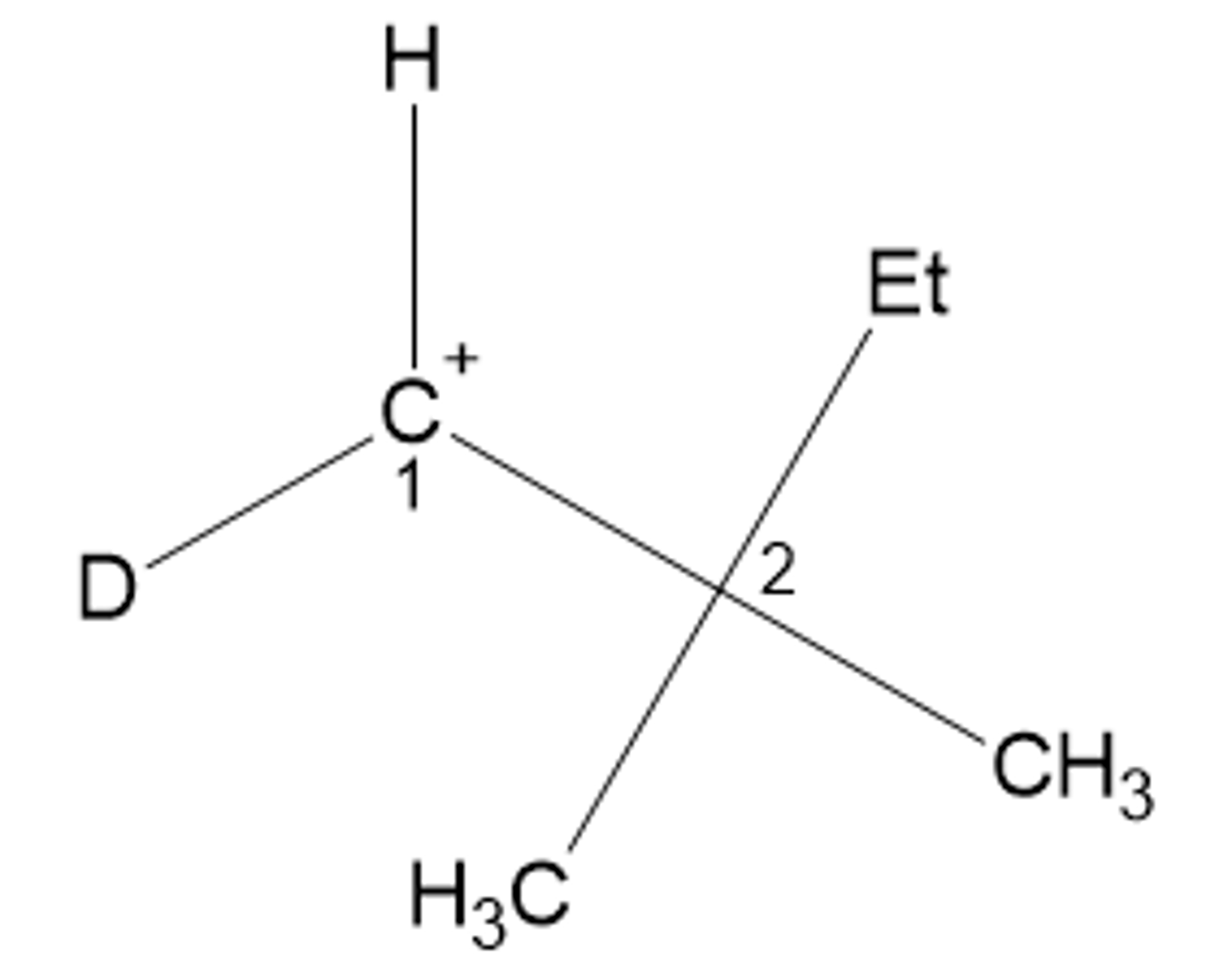
Neither. There are only 3 unique bonds (the 2 terminal methyls are equivalent).
R or S for the carbon labeled 2?
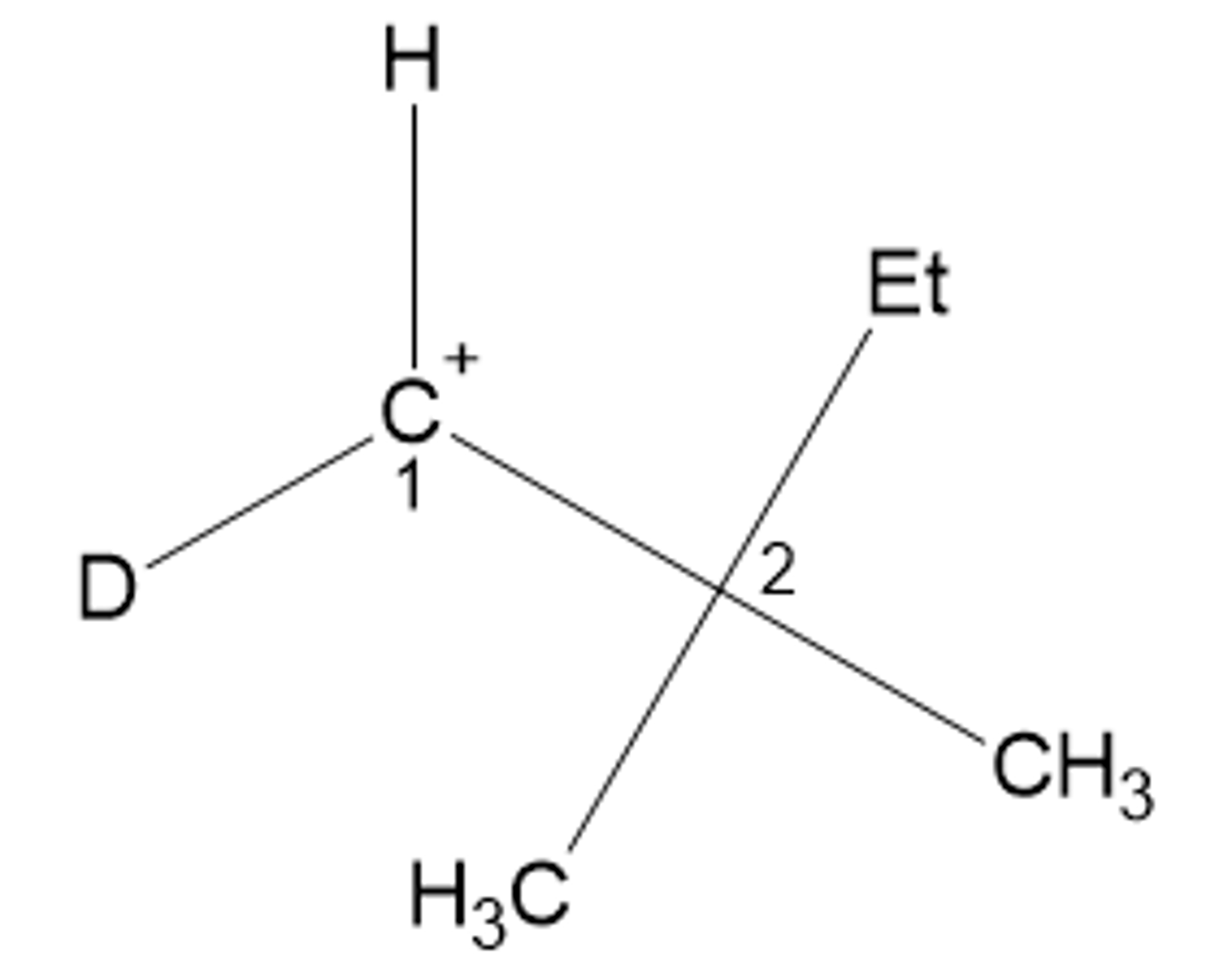
R.
Priority of groups:
1. OH
2. C=O
3. CH2OH
4. H
Note that the left and right groups are coming forward, and the lowest priority group must be in the back to determine R or S accurately.
R or S for the central carbon?
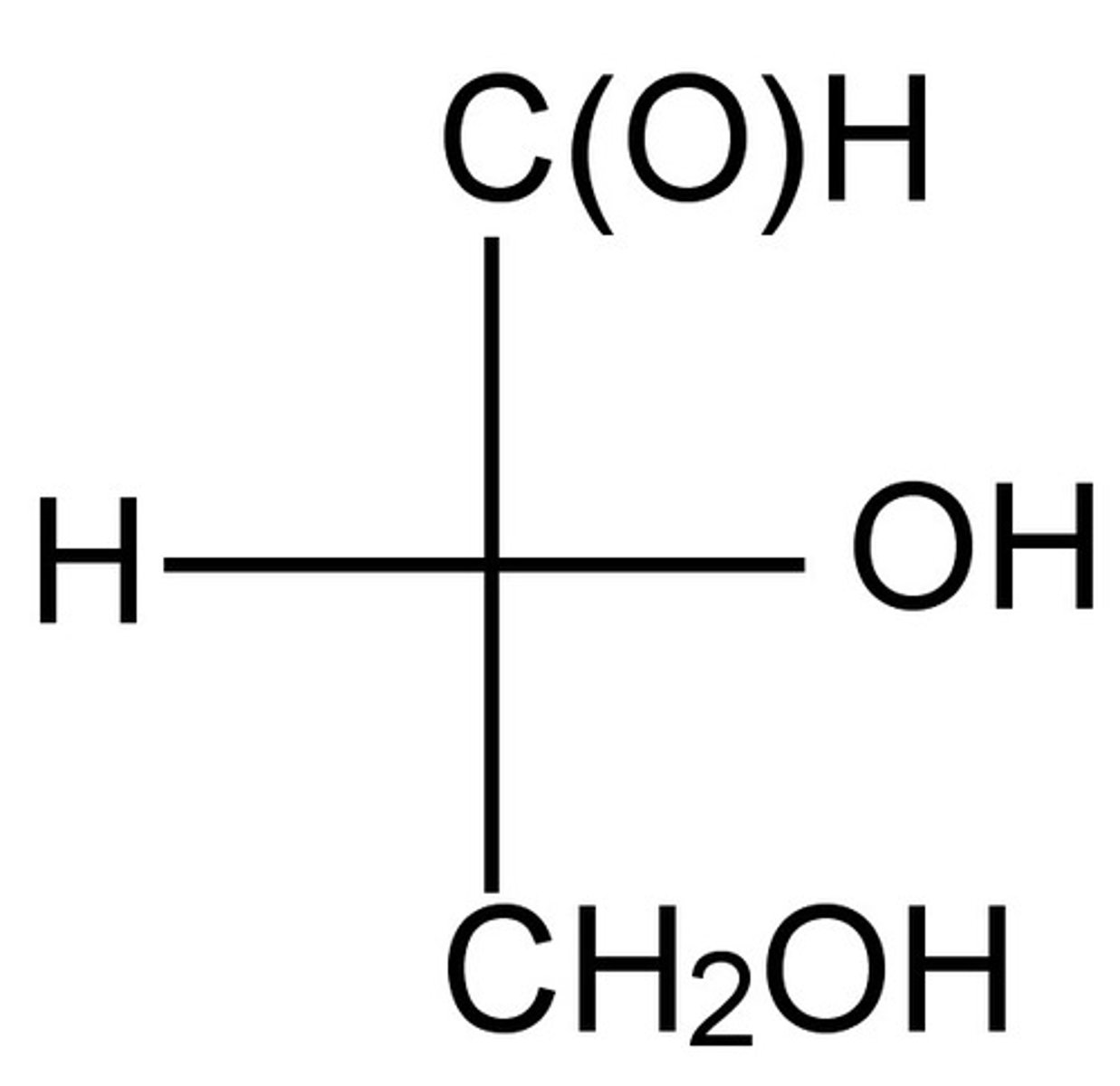
NOTE The R and S Practice is now over, and you should change your settings so that the Term side is shown first! This is so you can answer the rest of the cards as normal.
NOTE The R and S Practice is now over, and you should change your settings so that the Term side is shown first! This is so you can answer the rest of the cards as normal.
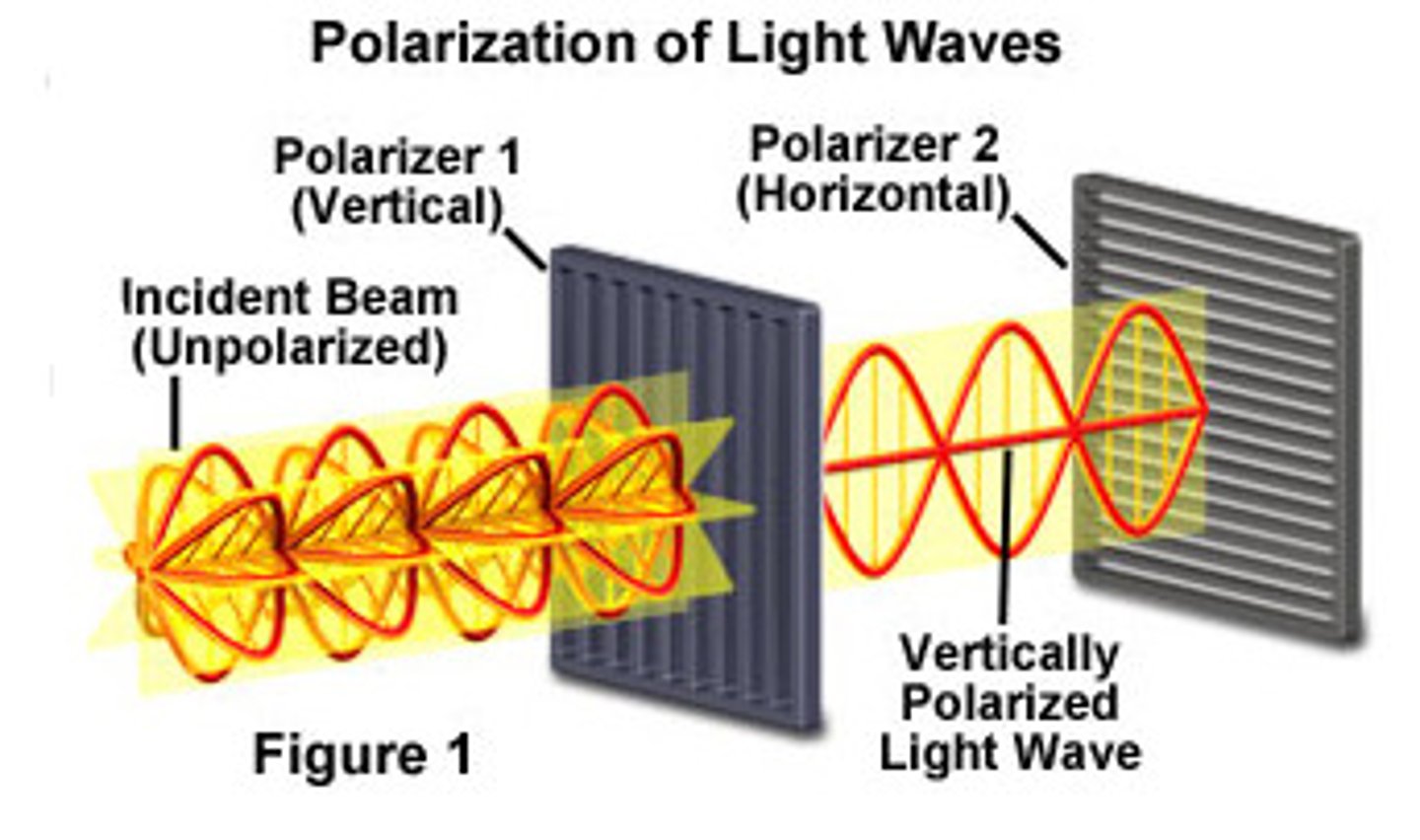
What is the difference between unpolarized light and plane-polarized light?
Plane-polarized light has all of its waves are oriented in the same direction while unpolarized light has waves oriented in all directions.
What happens to plane-polarized light as it passes through a polarimeter tube filled with an optically active substance?
The plane-polarized light will rotate.
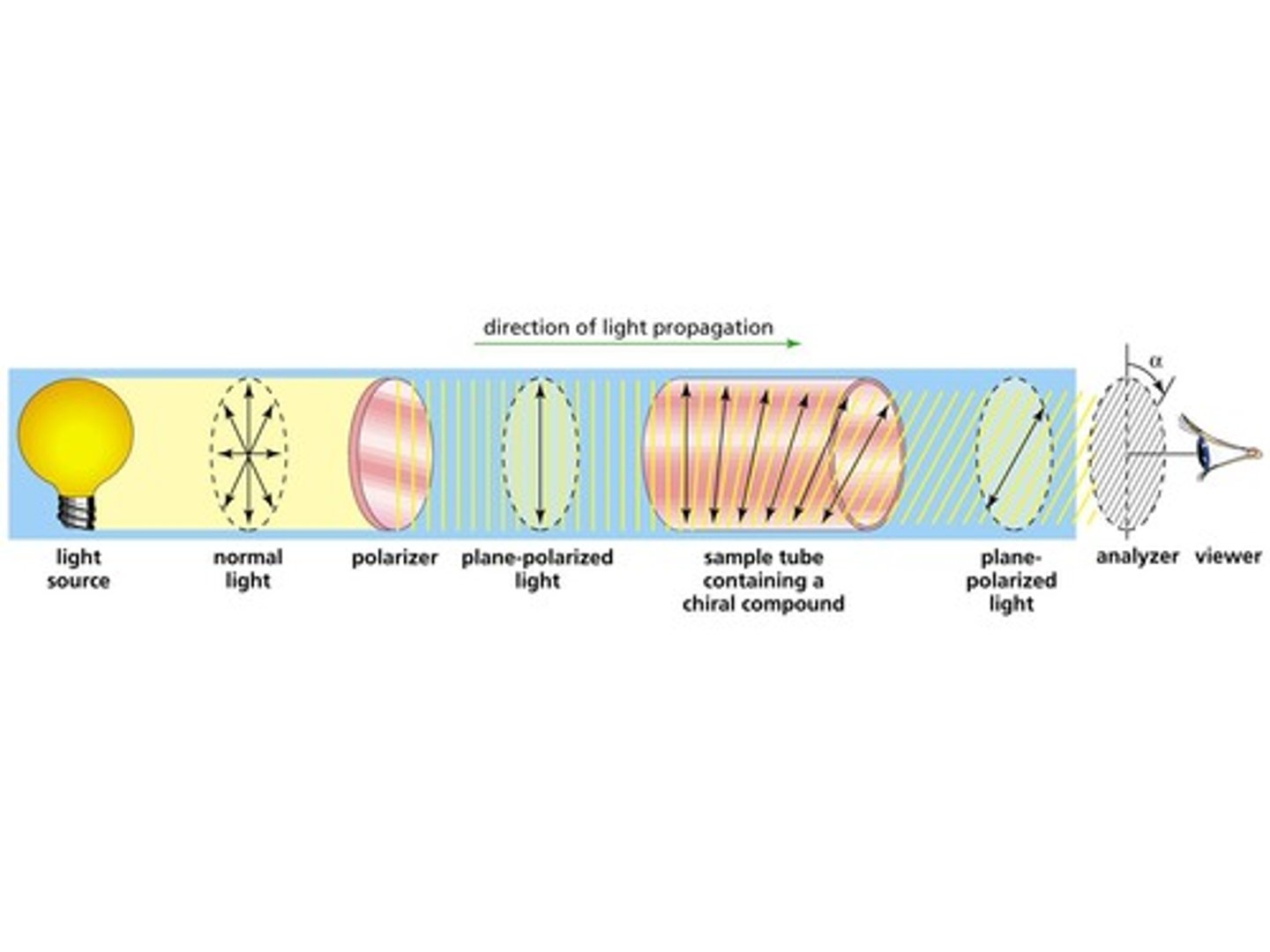
A scientist is determining the Observed Rotation (α) of a substance. How will the scientist use the polarimeter tube to determine this?
The scientist will rotate the analyzer until she observes light coming through the analyzer. At this point, the scientist would measure the degree to which the scientist rotated the analyzer.
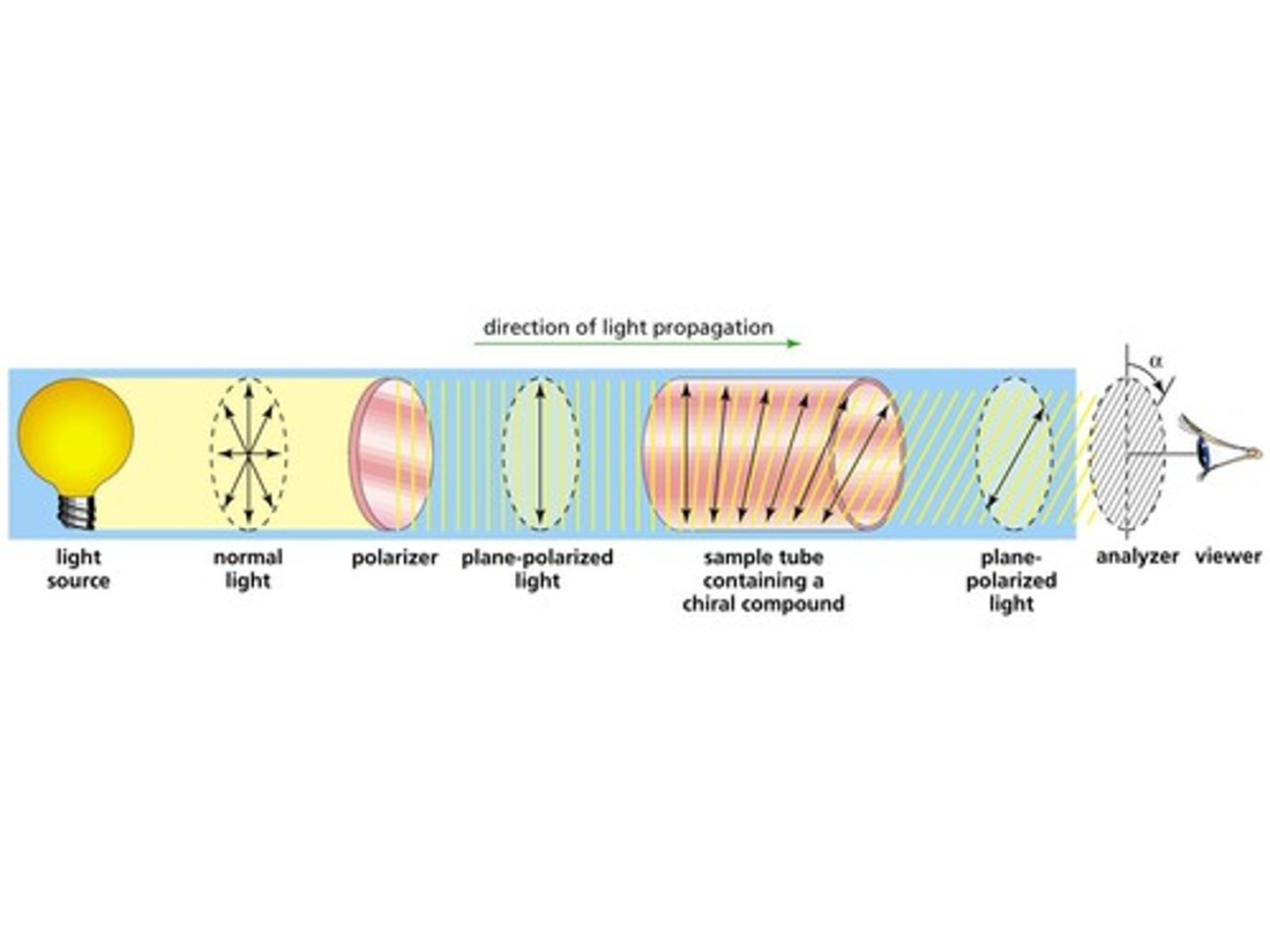
α is positive, which means the substance is ____________________ and it rotated the substance to the _____________.
α is negative, which means the substance is ____________________ and it rotated the substance to the _____________.
Fill in the blanks using the following terms:
- right
- left
- levorotatory (l)
- dextrorotatory (d)
α is positive, which means the substance is dextrorotatory (d) and it rotated the substance to the right.
α is negative, which means the substance is levorotatory (l) and it rotated the substance to the left.
You double the concentration of the substance in a polarimeter tube. What happens to the observed rotation (α)?
(A) It halves.
(B) It does not change.
(C) It doubles.
(D) It quadruples.
(C) It doubles.
You double the length of the polarimeter tube. What happens to the observed rotation (α)?
(A) It halves.
(B) It does not change.
(C) It doubles.
(D) It quadruples.
(C) It doubles.
What is the difference between α and [α]?
α is the observed rotation and is affected by concentration and
path length.
[α] is the absolute rotation and is independent of concentration and path length. A substance's absolute rotation is constant and does not change.
What is the equation for specific rotation in terms of observed rotation? Be sure to specify the units for each variable.
[α] = α / (c ⋅ l)
[α] = absolute rotation (degrees)
α = observed rotation (degrees)
c = concentration (g/mL)
l = path length (dm)
Struggling to keep your MCAT equations straight? Simply conquer the 100 most important equations using Andrew's 100 Most Essential Equations Mastery Course @ https://mcatselfprep.com/course/andrews-equation-mastery-course/
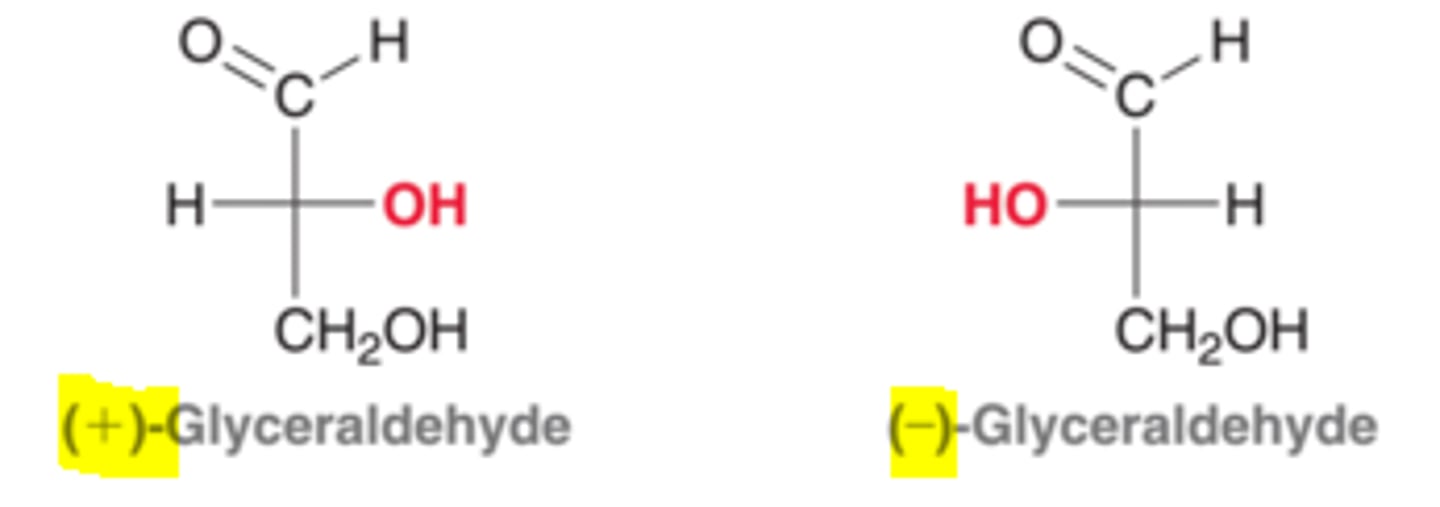
(+)-Glyceraldehyde (MM = 90.08) has a specific rotation of 62 degrees. If a 3.2 meter-long polarimeter contains a 143 mL solution containing 4.35⋅10^-3 moles of Glyceraldehyde, what will be the observed rotation?
(A) 5.44
(B) 35.84
(C) 78.23
(D) 657.98
(A) 5.44
3.2 meter ⋅ 10 dm / 1 m = 32 dm = l
4.35⋅10^-3 moles ⋅ 90.08 g / mole = approx. 360⋅10^-3 g (actual: 391.848⋅10^-3 g)
360⋅10^-3 g / 143 mL = approx. 2.5⋅10^-3 g/mL (actual: 2.74⋅10^-3 g/mL)
[α] = α / (c ⋅ l)
62 = α / (2.7⋅10^-3 ⋅ 32)
62 = α / (90⋅10^-3)
62 ⋅ 90⋅10^-3= α
α = approx. 5 (actual: 5.44)
Need help with MCAT math? Become an MCAT math wizard using Andrew's High-speed Math Mastery Course @ https://mcatselfprep.com/course/andrews-high-speed-math-mastery-course/
CRB If you had 2.7 moles of (+)-Glyceraldehyde and 1.2 moles of (-) Glyceraldehyde mixed in the same polarimeter, what would the rotation of light be comparable to?
(A) 3.9 moles of (+)-Glyceraldehyde
(B) 1.5 moles of (+)-Glyceraldehyde
(C) No rotation of light at all
(D) Not enough information to determine
(B) 1.5 moles of (+)-Glyceraldehyde
The 1.2 moles of (-)-Glyceraldehyde would cancel out the rotation of 1.2 moles of the (+)-Glyceraldehyde. This means the effects of the remaining 1.5 moles of (+)-Glyceraldehyde could be seen.
CRB True or false? Based on the previous card's reasoning, a 50-50 mixture of the two enantiomers would have no net observed rotation of light.
True. Based on the previous card's reasoning, a 50-50 mixture of the two enantiomers would have no net observed rotation of light.
The term for this type of mixture is "racemic".
(+)-Glyceraldehyde has a specific rotation of 62 degrees. What is the specific rotation of (-)-Glyceraldehyde?
(A) 124
(B) 62
(C) -62
(D) -124
(C) -62
+ and - isomers rotate light to the same degree in the opposite direction.
True or False. There is no need to use a polarimeter if you are able to view the chemical structure of a compound.
False. + and - distinctions are not directly related to R and S distinctions. + and - distinctions must be determined experimentally.
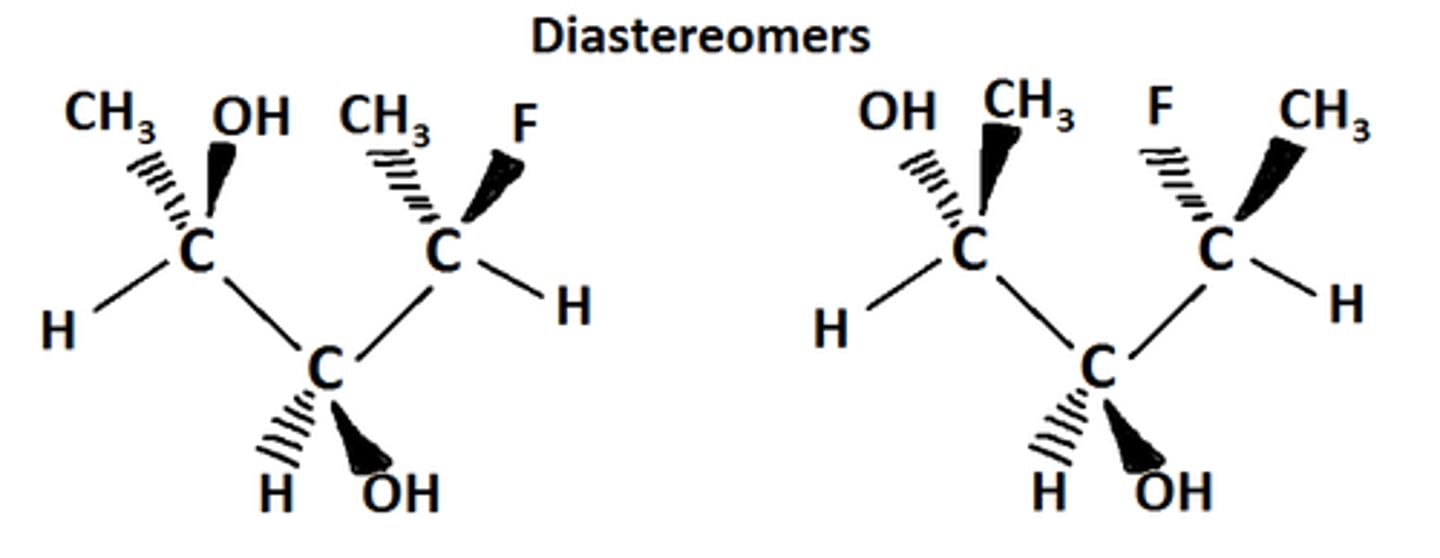
What is the difference between enantiomers and diasteriomers?
Enantiomers are stereoisomers that are nonsuperimposable mirror images and have opposite configurations at ALL chirality centers.
Diasteriomers are stereoisomers that are nonsuperimposable NON-mirror images and have opposite configurations at SOME chirality centers.
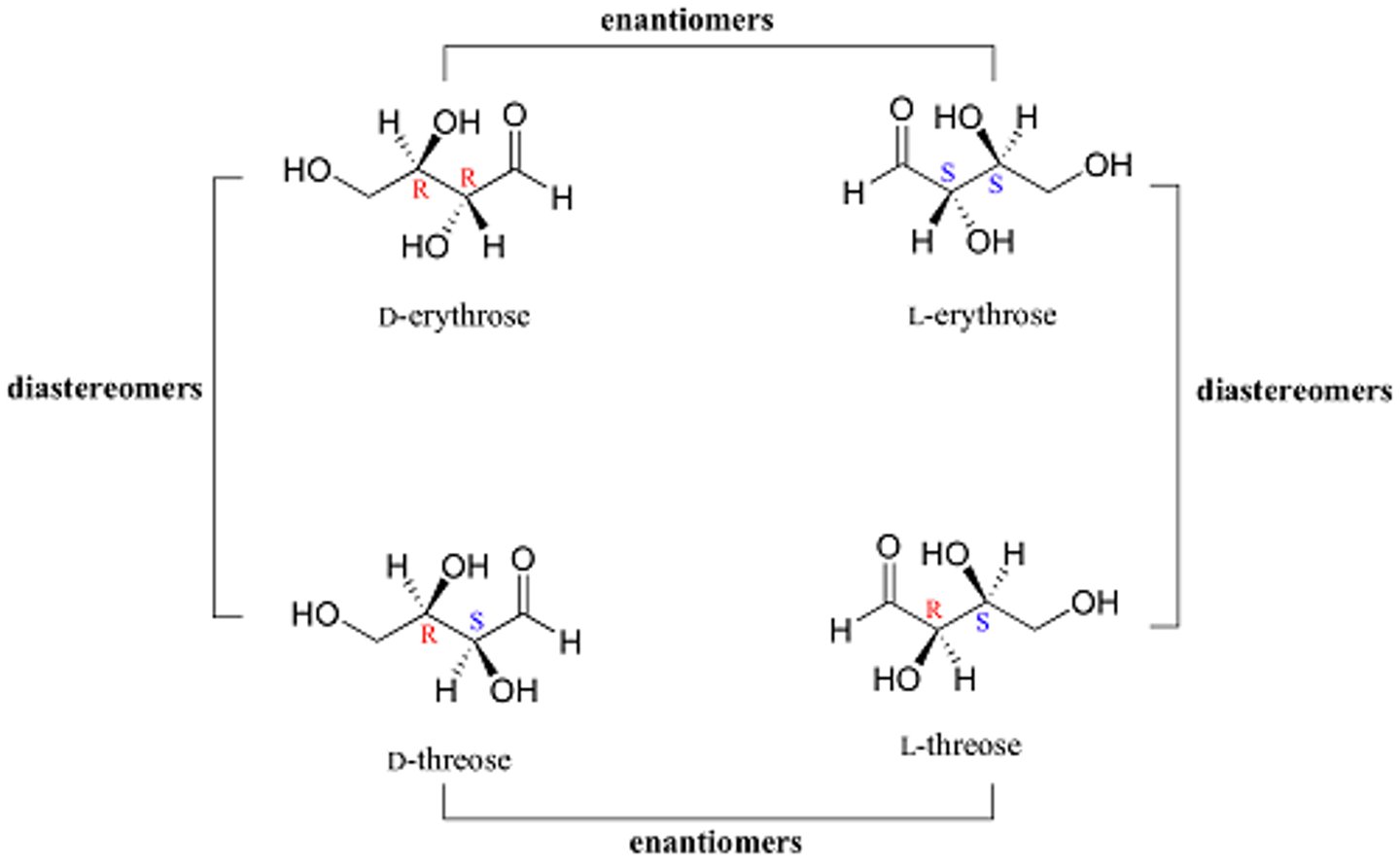
CRB Based on the previous card, which of the following physical properties can vary between diastereomers?
I. Molecular Weight
II. Melting Point
III. Boiling Point
(A) II only
(B) I and II only
(C) II and III only
(D) I, II and III
(C) II and III only
Diastereomers can have different physical properties, including melting and boiling points.
They are still stereoisomers, however, so their molecular weights will be equal.
Take this multiple choice quiz to practice these stereochemistry concepts: http://highered.mheducation.com/sites/0072397462/student_view0/chapter5/multiple_choice_quiz.html
Take this multiple choice quiz to practice these stereochemistry concepts: http://highered.mheducation.com/sites/0072397462/student_view0/chapter5/multiple_choice_quiz.html
CRB Which of the following is a type of diastereomer where the chirality only differs at one chirality center?
(A) Enantiomer
(B) Epimer
(C) Anomer
(D) Meso Compound
(B) Epimer
An epimer is a type of diastereomer where the chirality only differs at one chirality center.
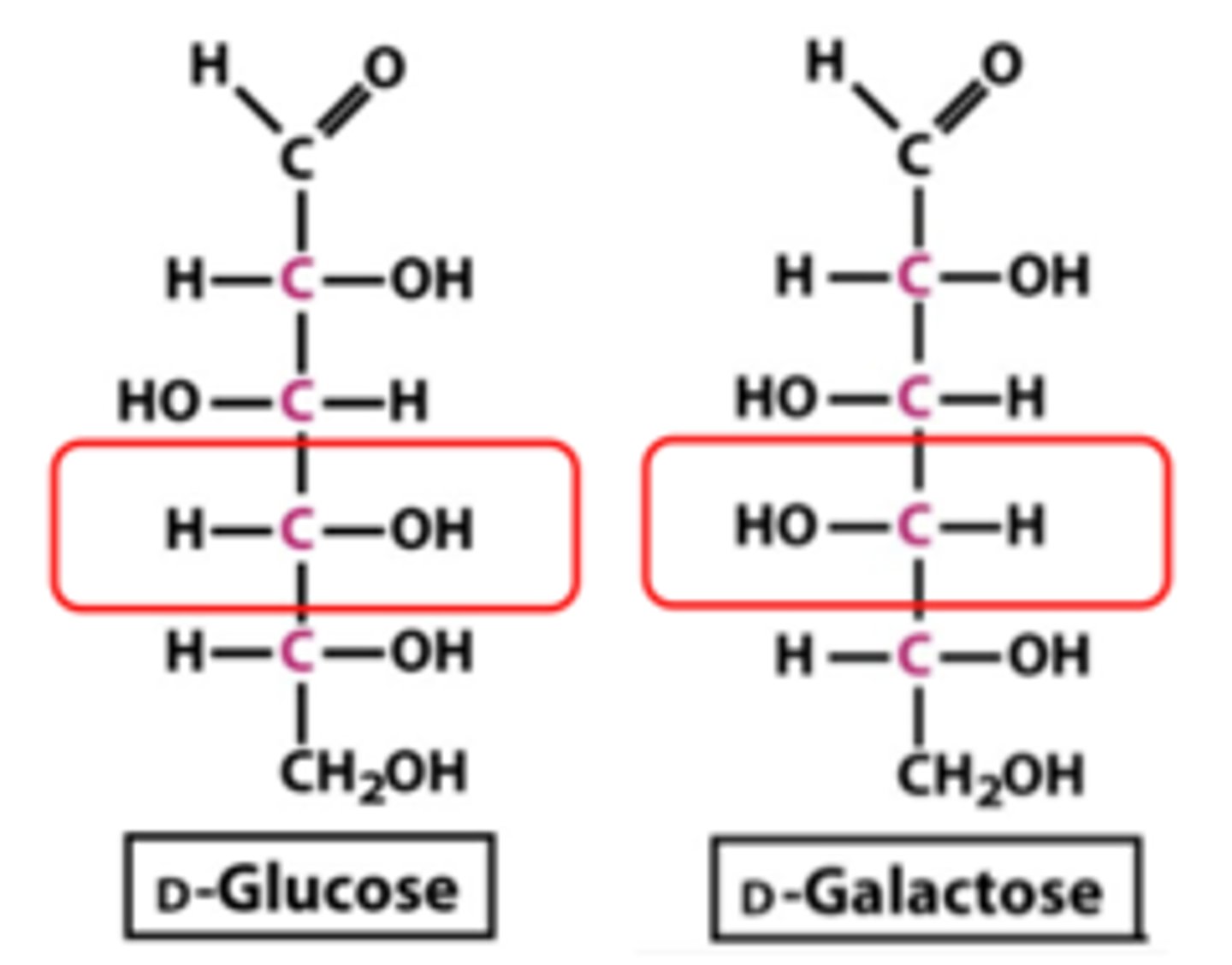
CRB Which of the following is a type of epimer that is formed depending on how a ringed structure forms?
(A) Diastereomer
(B) Enantiomer
(C) Anomer
(D) Meso Compound
(C) Anomer
An anomer is a type of epimer that forms depending on how a ringed structure forms.
Practice assigning trans vs cis using the following practice questions (scroll to the very bottom of the webpage): https://www.chemistrysteps.com/cis-and-trans-stereoisomerism-in-alkenes/
Check your answer here (scroll to the very bottom of the webpage): https://www.chemistrysteps.com/cis-and-trans-stereoisomerism-in-alkenes/
When do you assign the Z designation to an alkene? E designation?
Assign the Z designation when the high-priority groups are on the same side (Zame)
Assign the E designation when the high-priority groups are on opposite sides (oppositE)
Complete the following practice problems to practice assigning Z and E designations: http://www.chemguide.co.uk/basicorg/questions/q-eznotation.pdf
Check your answers here: http://www.chemguide.co.uk/basicorg/questions/a-eznotation.pdf
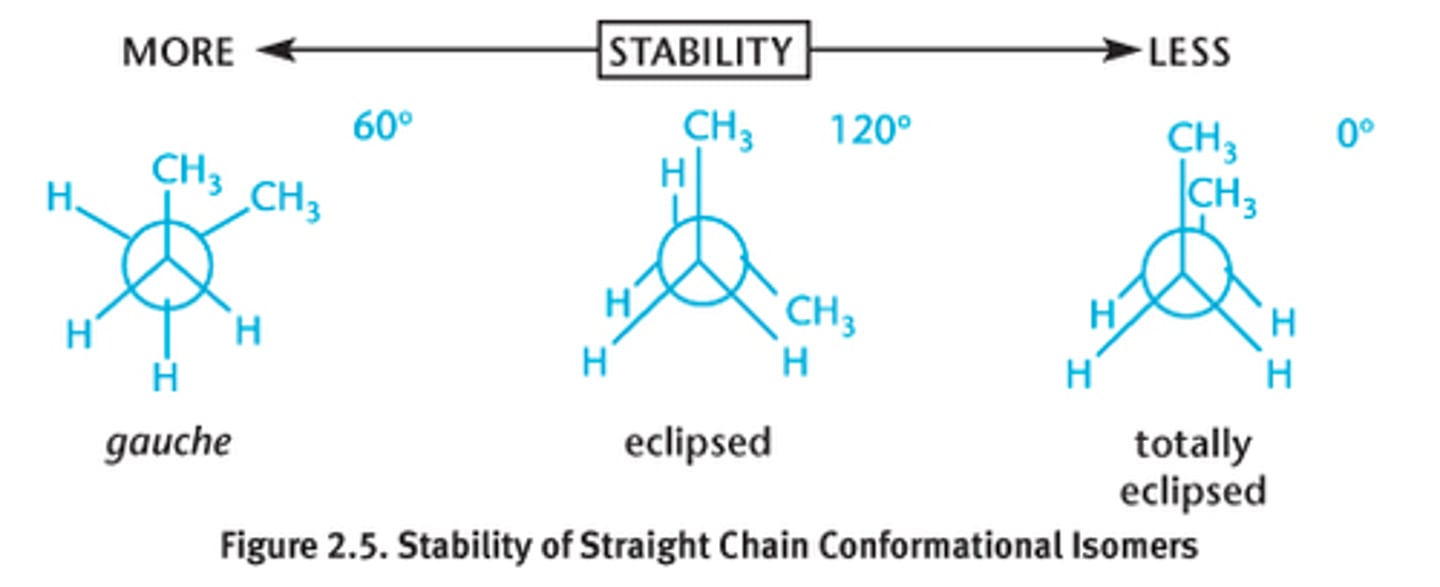
What is the difference between Constitutional Isomers and Conformational Isomers?
Constitutional Isomers have the same make up but a different arrangement of bonds.
Conformational Isomers have the same make up, the same arrangement of bonds, but their bonds are rotated differently from one another (see image).
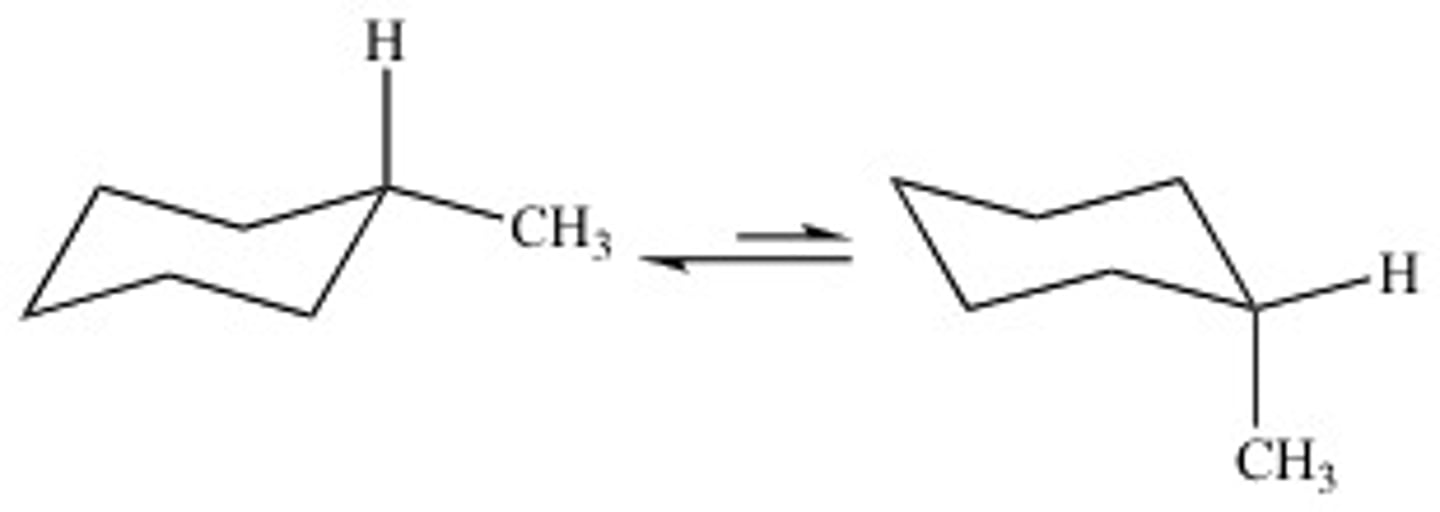
CRB True or false? Conformational isomers can be interconverted by simply rotating bonds, meaning that the molecules are the same and will have all of the same chemical and physical properties.
True. Conformational isomers can be interconverted by simply rotating bonds, meaning that the molecules are the same and will have all of the same chemical and physical properties.
Imagine a Newman Projection. How does it compare to a wedge and dash depiction of a molecule?
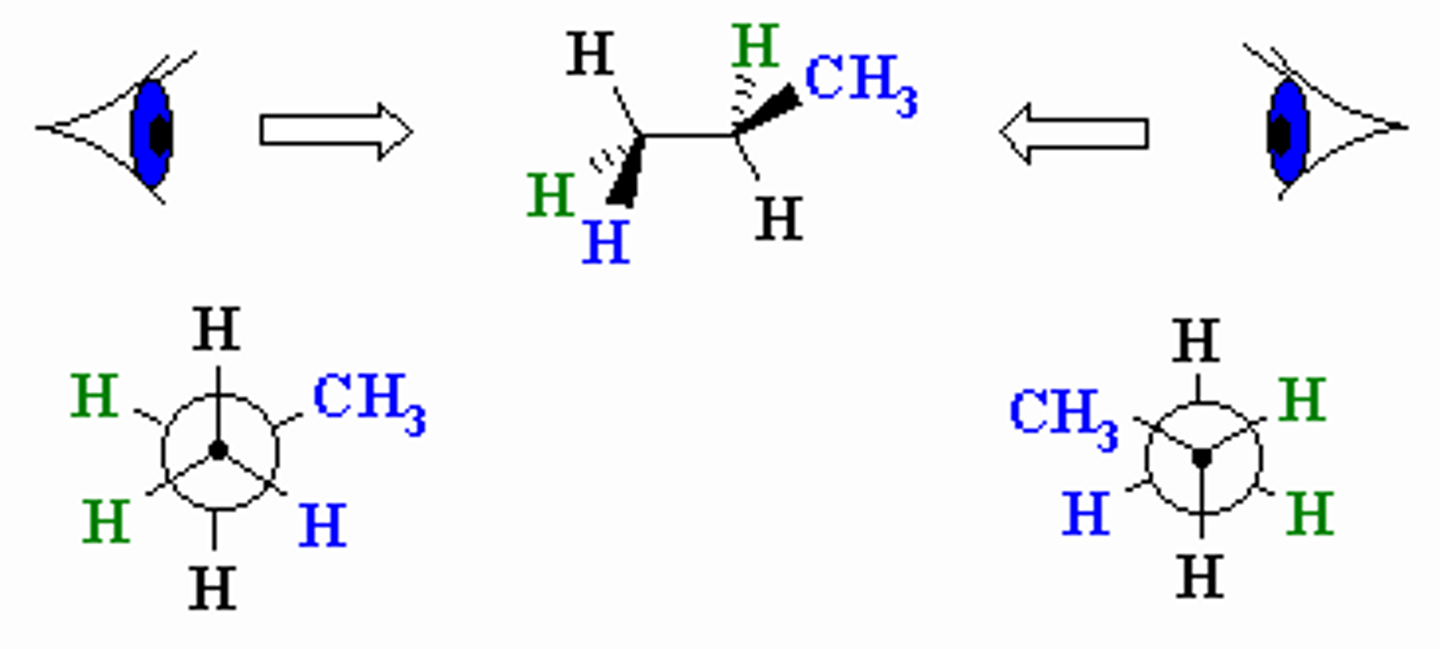
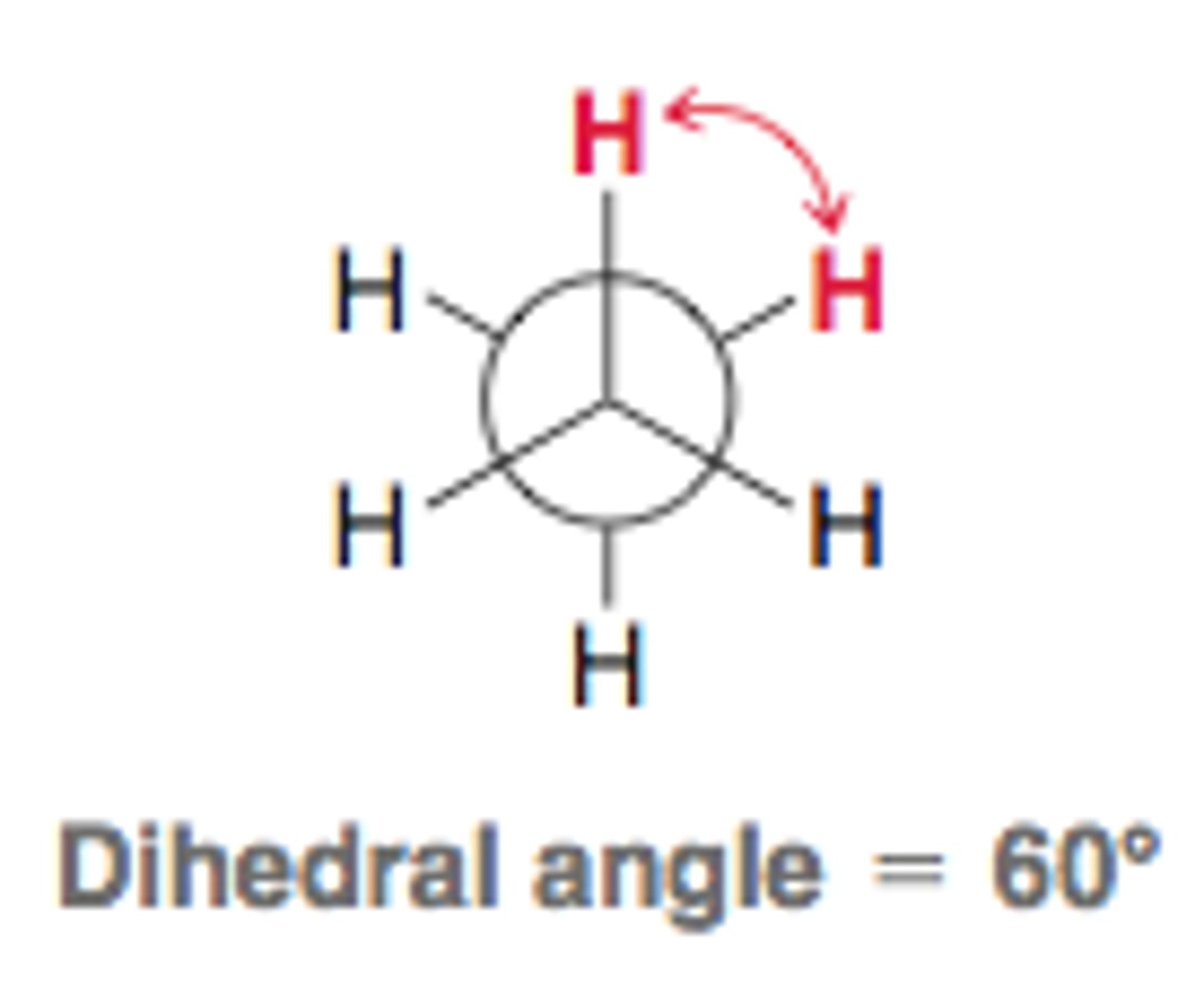
Dihedral/Torsional Angle is the angle between which substituents?
(A) The opposing substituents on the close and distant carbon of a Newman Projection.
(B) The neighboring substituents on the close and distant carbon of a Newman Projection.
(B) The neighboring substituents on the close and distant carbon of a Newman Projection.
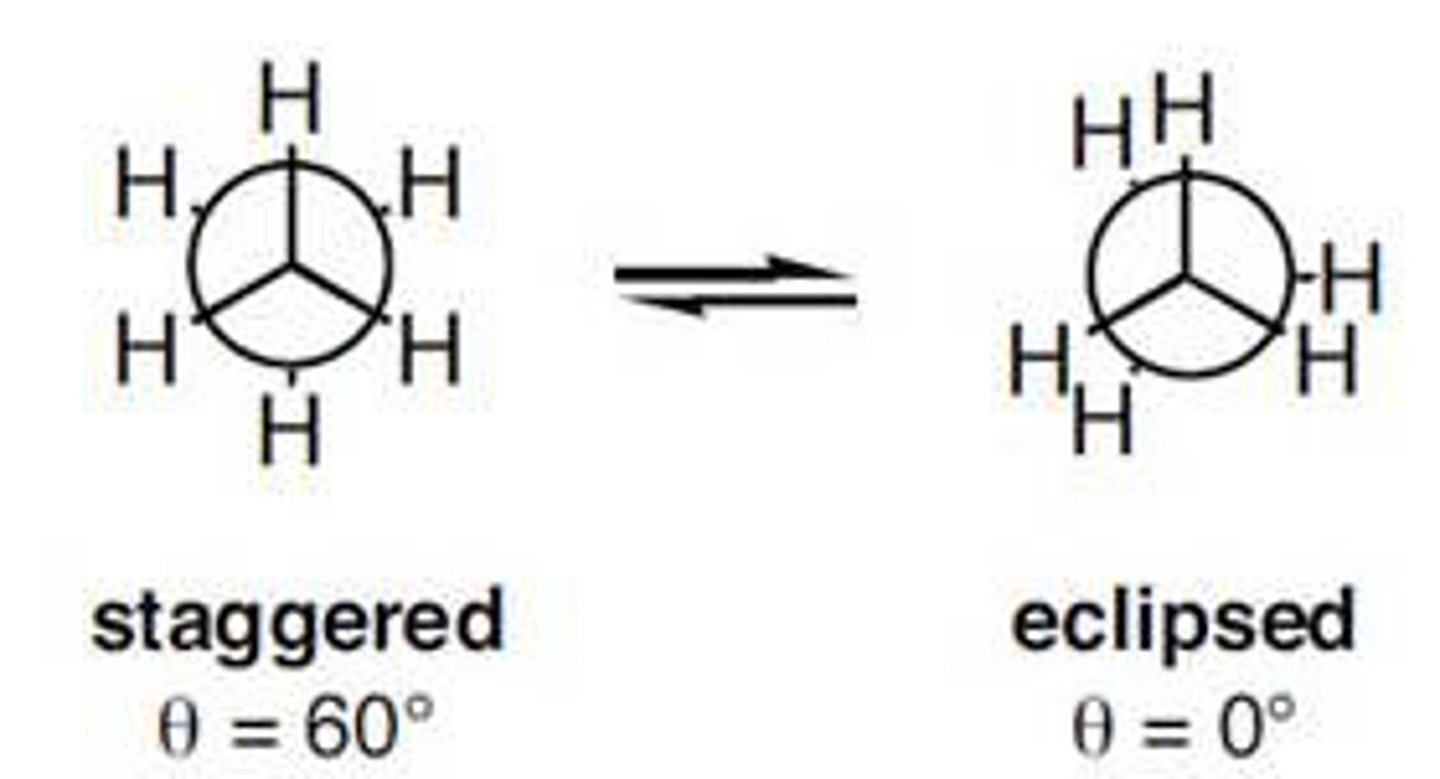
Draw a Staggered vs. Eclipsed Newman Projection
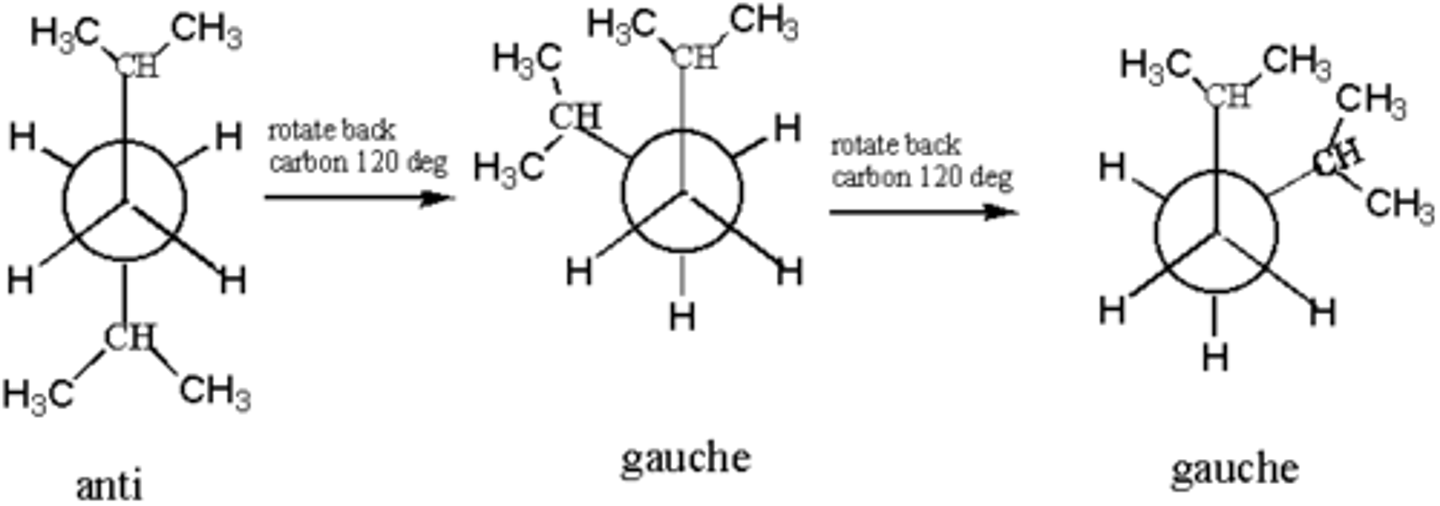
CRB Draw an Anti vs. Gauche Newman Projection
Label the following energy diagram: http://d2vlcm61l7u1fs.cloudfront.net/media%2F2dc%2F2dc7f7ff-f1a6-4084-a0f6-46f3cfa30030%2FphpipIlYf.png
See image for answers.
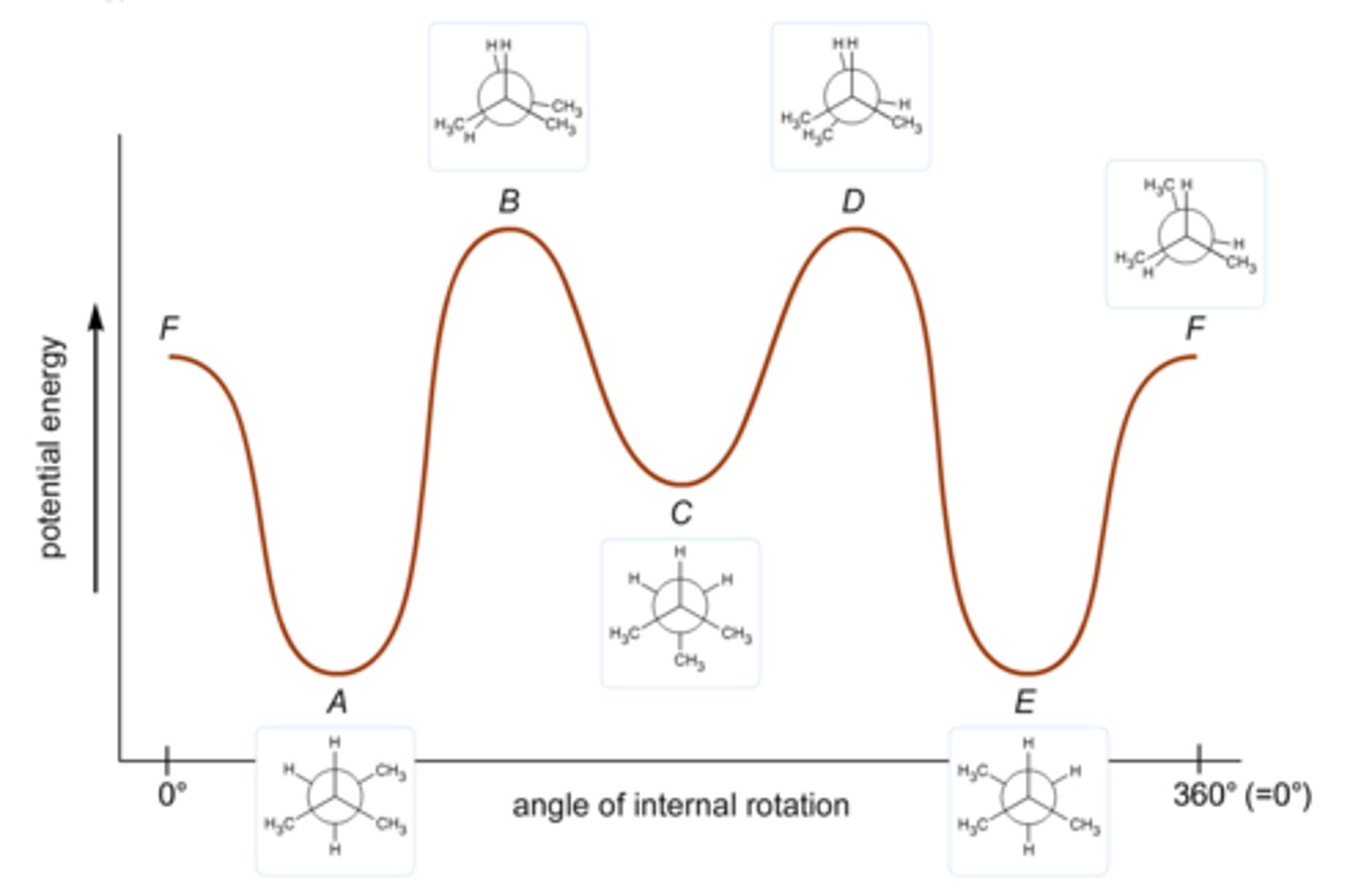
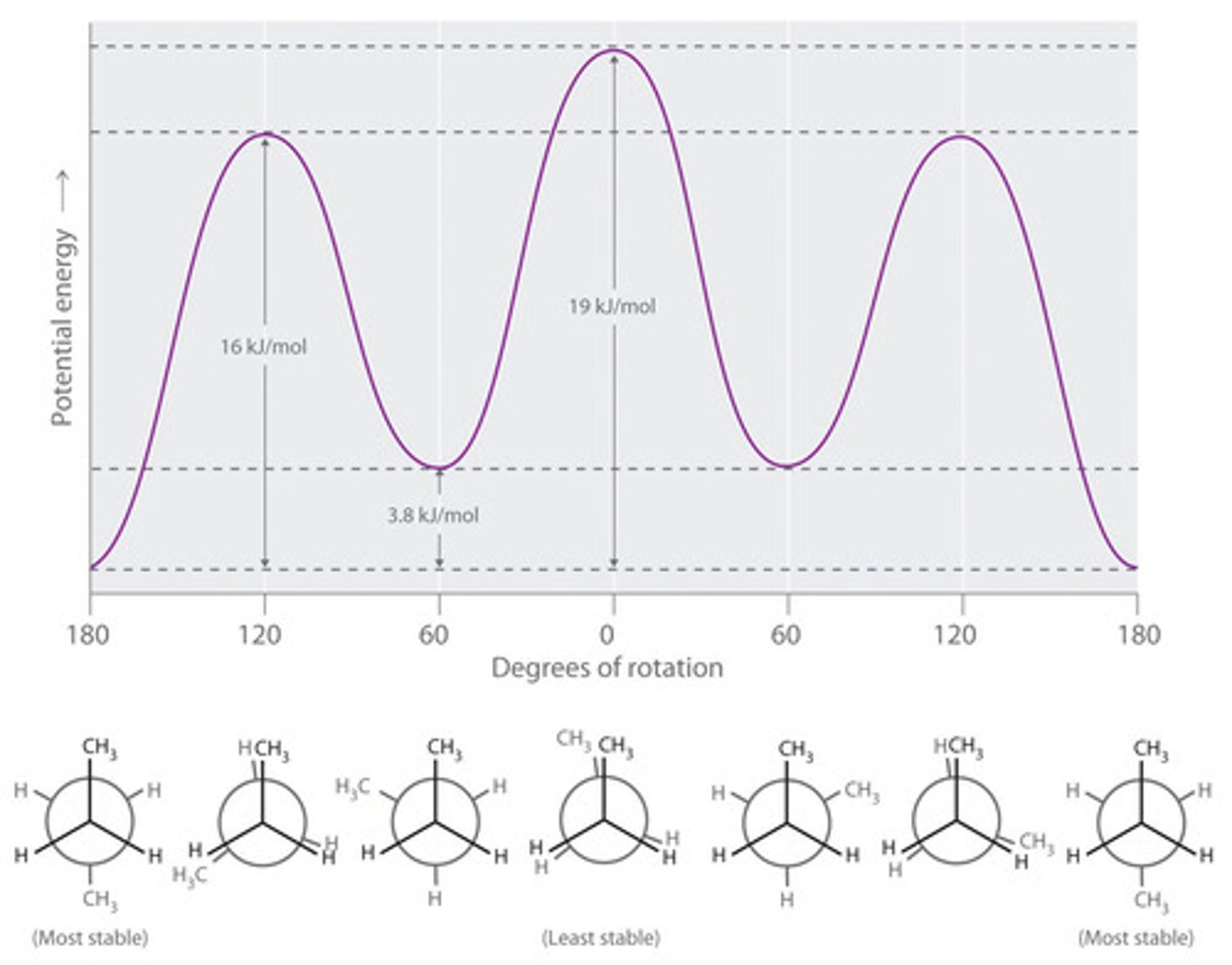
Draw the energy diagram for butane at each of its six Newman projection conformations.
See image for answer.
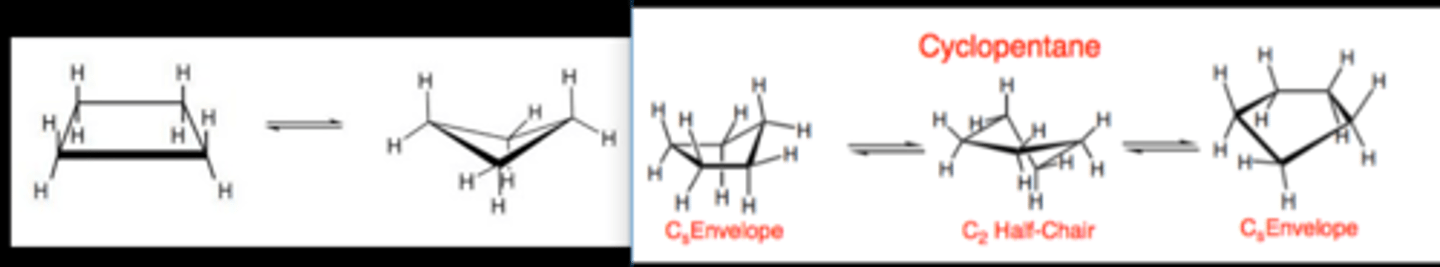
CRB Cycloalkanes are very common, and often have a preferred cyclic conformation to reduce ring strain if below six carbons. Draw out the "puckered" formation of cyclobutane and the "envelope" conformation of cyclopentane.
Cyclobutane on left, Cyclopentane on right.
CRB Recall from the previous lesson that the ideal bond angle for an sp3-hybridized (tetrahedral) Carbon is 109.5 degrees. Because of ring strain, which of the following cycloalkanes will easily undergo hydrogenation?
I. Cyclopentane
II. Cyclobutane
III. Cyclopropane
(A) I only
(B) I and III only
(C) II and III only
(D) I, II and III
(C) II and III only
Cyclopropane (60 degree bond angles) and cyclobutane (90 degree bond angles) exhibit large ring strain, and will readily undergo hydrogenation.
CRB Cyclohexane is the most commonly tested cycloalkane on the MCAT. Draw its "chair", "boat" and "twist-boat" conformations, and predict which conformations have the highest and lowest energy.
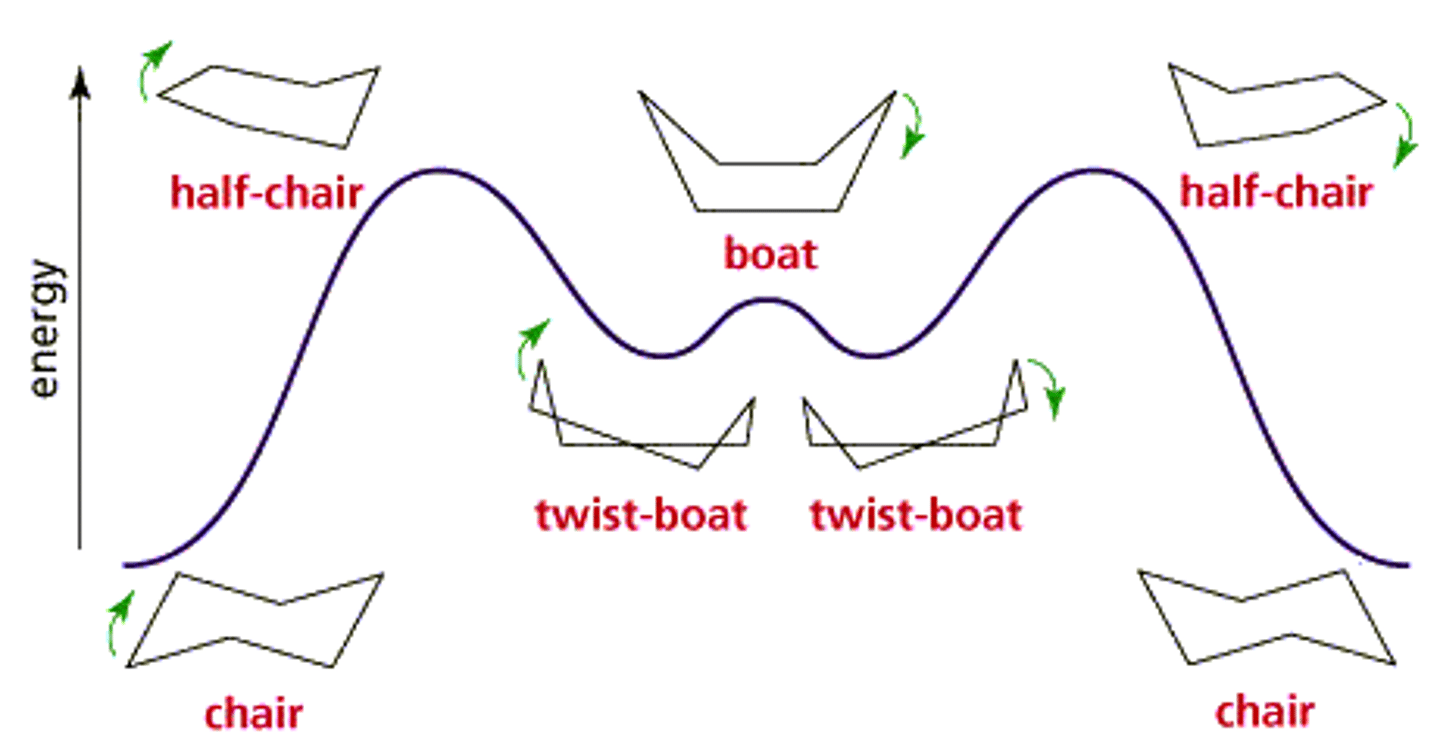
CRB Fill in the blanks: In its "chair" conformation, larger groups will want to be __________ to the ring to prevent gauche interactions, meaning that hydrogens should be _________.
(A) Axial, Equatorial
(B) Axial, Horizontal
(C) Horizontal, Axial
(D) Equatorial, Axial
(D) Equatorial, Axial
In its "chair" conformation, larger groups will want to be equatorial to the ring to prevent gauche interactions, meaning that hydrogens should be axial.