Quantum Mechanics (Module 1)
1/20
Earn XP
Description and Tags
ch 5
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
Quantum Mechanics
Study of the motion of sub-atomic particles
matter waves (phenomenon)
aka de broglie waves
particles when moving exhibit wave like properties
Energy equations
energy of light wave and light particle
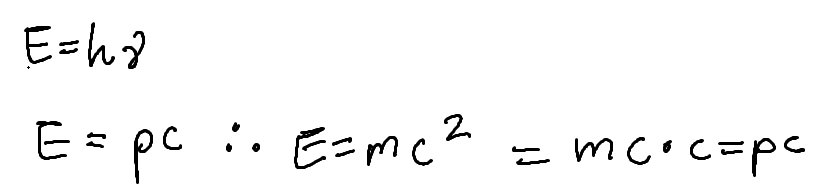
relation of wave nature and particle nature
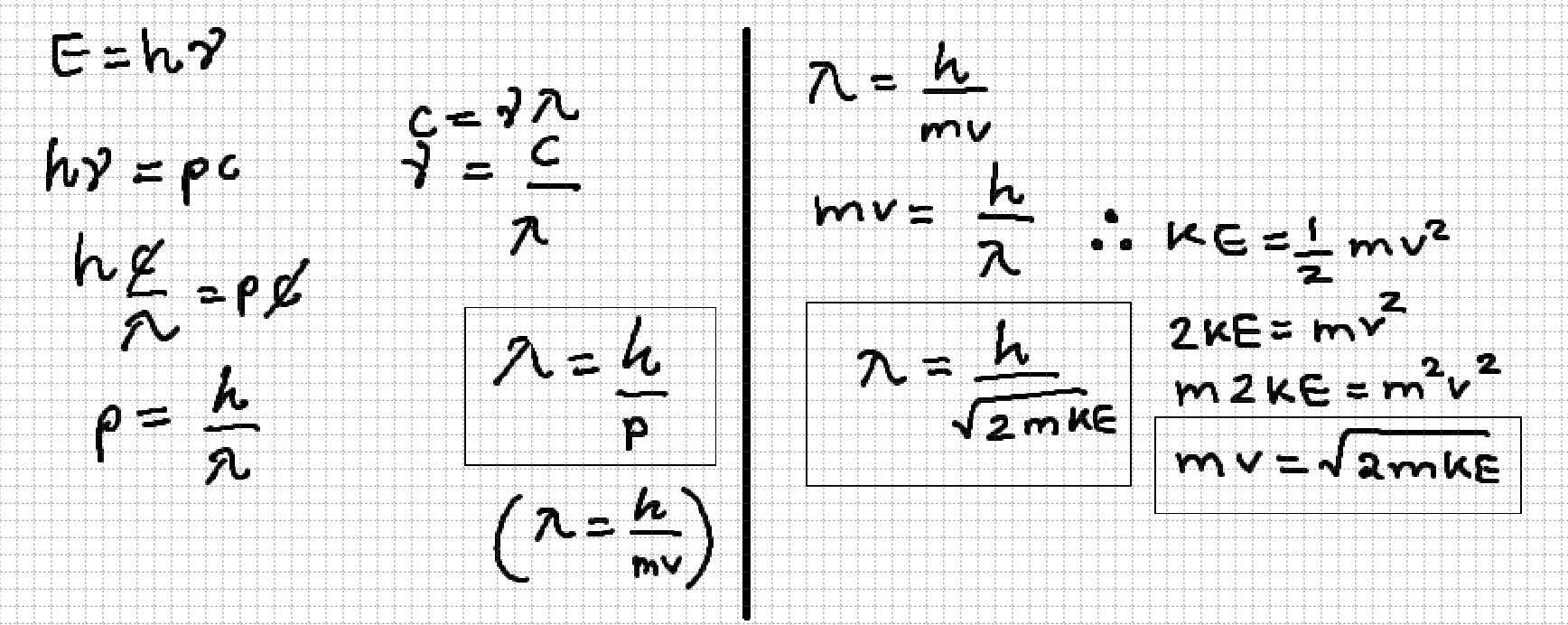
Planck’s constant
6.626×10-34 m2kg/s
accelerate particles
To accelerate a particle an external potential is applied. The applied external potential is proportional/related to the energy of the particle.
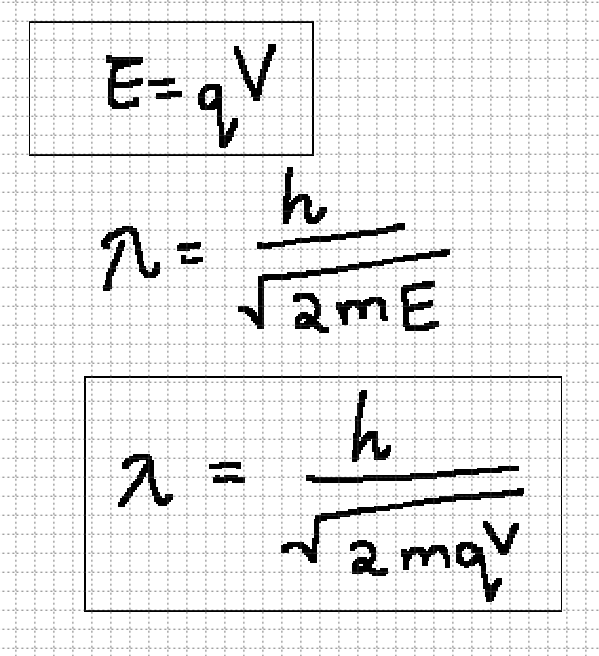
Heisenberg’s Uncertainity Principle
It is impossible to find the position and momentum of a particle simultaneously.
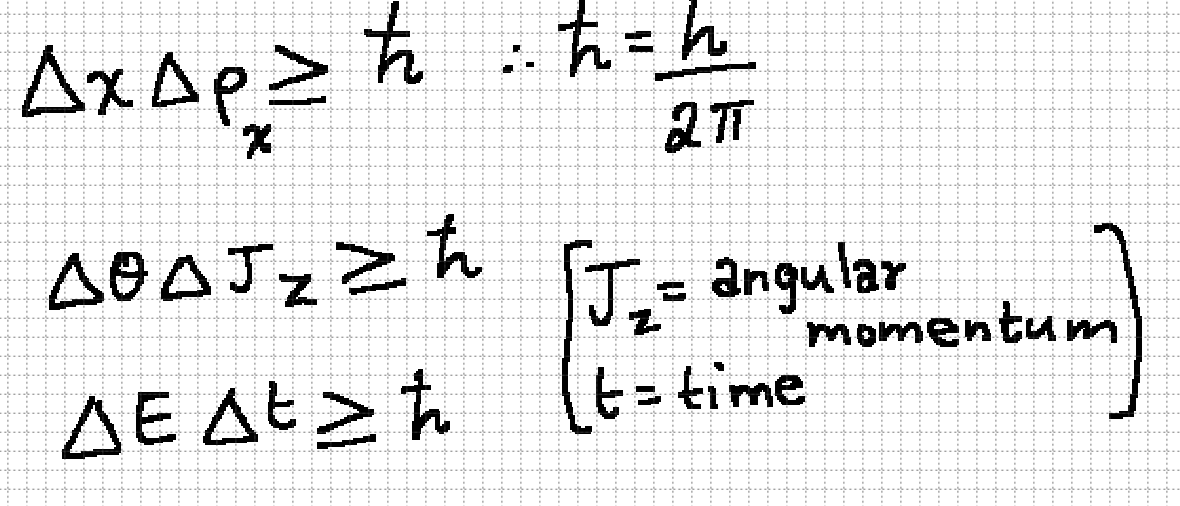
Canonically conjugate variables
position and momentum
angular displacement and angular momentum
energy and time
radius of the nucleus
10-14 m
applications of uncertainity principle
Absence of e- in the nucleus
Uncertainity of frequency of light emitted by the atom
Natural line broadening
Absence of electrons in the nucleus
Uncertainity of the frequency of the light emitted by the atom
Natural Line broadening
Probability of finding the particle in unit volume
Probability density of a volume
Normalization condition
If a particle is definitely present within the probablity density it can be equated to 1.
general wave representation
wave function of wave propagating with momentum p (derive) in the x-direction
Schrodinger’s Equation (derive) Time dependent
Schrodinger’s Equation (derive) Time Independent
Particle in a Box