Energy - forms of energy/energy conversion
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
what are the two types of energy and give examples?

what is energy conversion? (edit this one)
A great example of how energy can be converted into different forms surrounds us in our everyday lives.
Plants use photosynthesis to convert light energy into chemical energy which they store in their leaves, stems and roots. This chemical process of photosynthesis is given by the following chemical reaction
Animals then eat these leaves and convert the chemical energy into heat and mechanical energy.
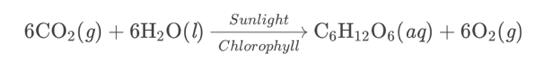
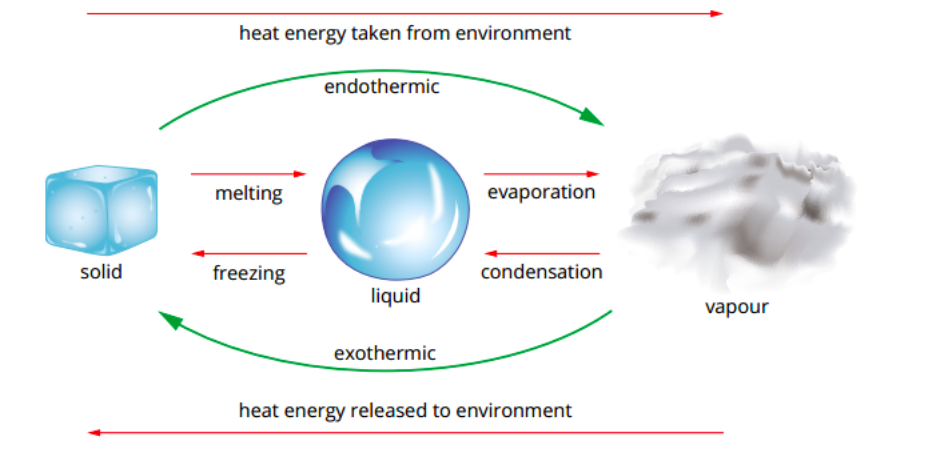
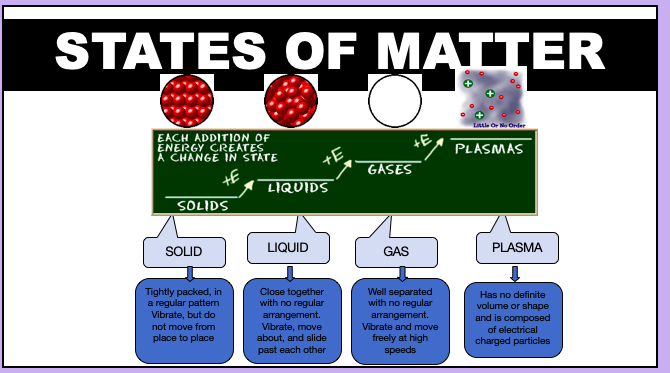
phase changes (4 states of matter) (need to explain these)
ØBased upon particle arrangement
ØBased upon energy of particles
ØBased upon distance between particles
phase changes: what are the diff types? (MF CVS)
melting
freezing
vaporisation (boling/evaporation)
condensation
sublimation
describe each of the phase changes
melting | freezing | vaporisation(evap/boil) |
solid to liquid | liquid to solid | liquid to gas |
heat goes into the solid as it melts | heat leaves the liquid as it freezes | heat goes into liquid as it vaporises |
condensation | sublimation | |
gas to liquid | solid to gas | |
Heat leaves the gas as it condenses. | Heat goes into the solid as it sublimates. |
phase diagrams (water changing state)
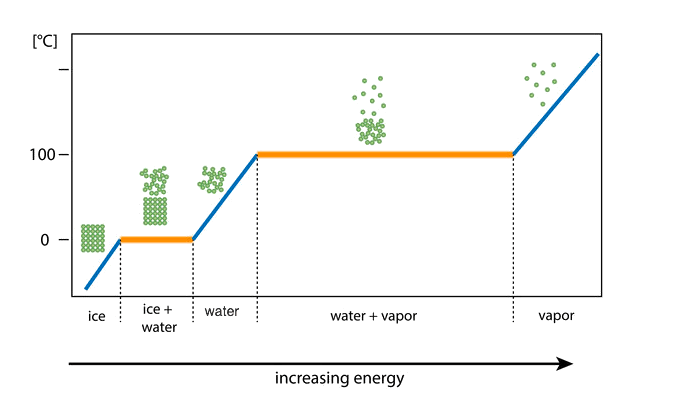
You can see that as energy is added the temperature increases.
At the melting point (0∘C), the water turns from a solid to a liquid.
At the boiling point (100∘C), the water turns from a liquid to a gas.
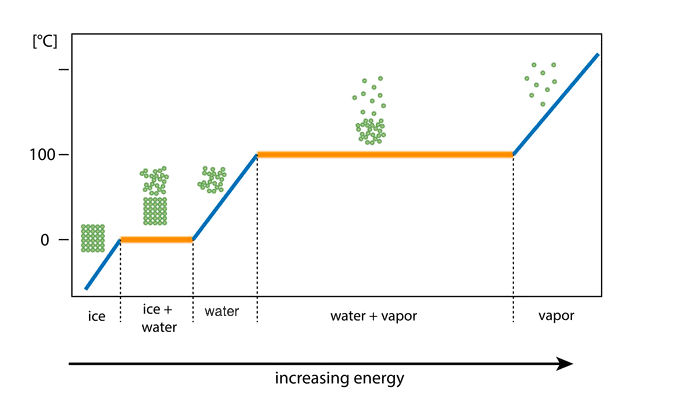
phase diagram: water changing state: temp
Notice how the temperature of the water does not change while it is changing state, even though energy is still being added
(Look at the yellow lines: they are flat!)
This is because the added energy is going into breaking bonds!
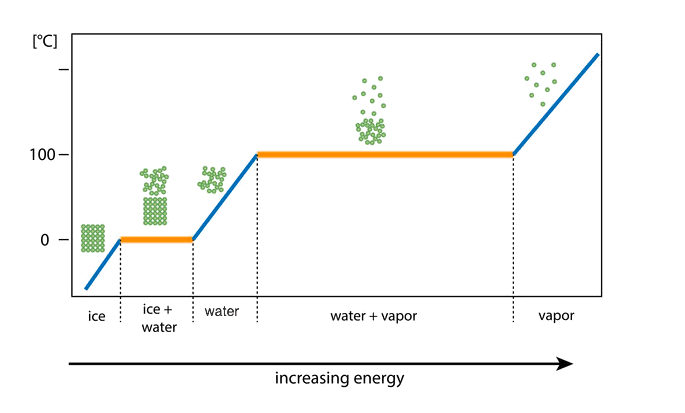
phase diagram: water changing state: energy added
As you add energy to a substance its temperature will rise.
This continues until the temperature reaches the melting or boiling points and the substance starts to change state.
Energy is still being added during the state change, but this energy is used to break bonds and does not change the temperature.
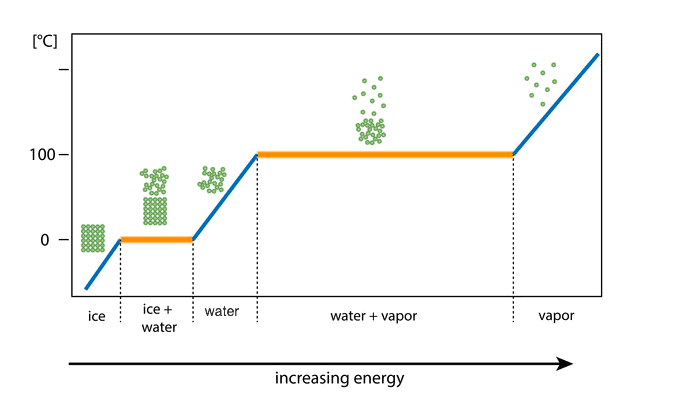
phase diagram: water changing state: reversing it
The same thing happens in the reverse too.
As you remove energy, the temperature of the water decreases.
When the water starts to change state, the energy of the water keeps dropping but the temperature stays still.
This is because the loss of energy results in the reforming of strong bonds instead of changing the temperature of the substance.
state changes: what do they require
State changes require energy to be added to or removed from a system. Melting and vaporisation (evaporation or sublimation) both require an energy input. Condensation and solidification (freezing or deposition) both require energy to be removed from the system.
what is latent heat
Energy added during a state change is called the latent heat.
The specific latent heat of fusion is the energy needed to change 1 kg of a substance from solid to liquid.
The specific latent heat of vaporisation is the energy needed to change 1 kg of a substance from liquid to gas.
difference between boiling and evaporation
Evaporation occurs only on the surface of the liquid, whereas Boiling occurs over the large mass of the liquid.
also depends on nature of liquid (e.g viscosity/ intermolecular bonding)
evaporation is slower, occurs only from the surface of the liquid, does not produce bubbles, and leads to cooling. Boiling is faster, can occur throughout the liquid, produces lots of bubbles, and does not result in cooling.
e.g to kill bacteria/purify water, you would boil it instead of just leaving it and evaporating it.
describe the changes of states with addition of energy
what is the relationship between kinetic energies and particles?
At any particular temperature, the particles in a substance have a range of kinetic energies.
most of the particles have similar kinetic energies,some particles have high energy or low energy.
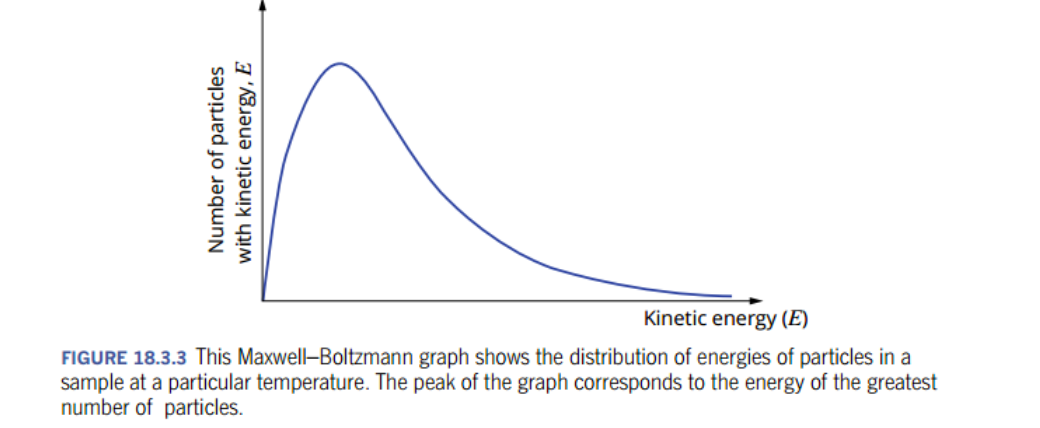
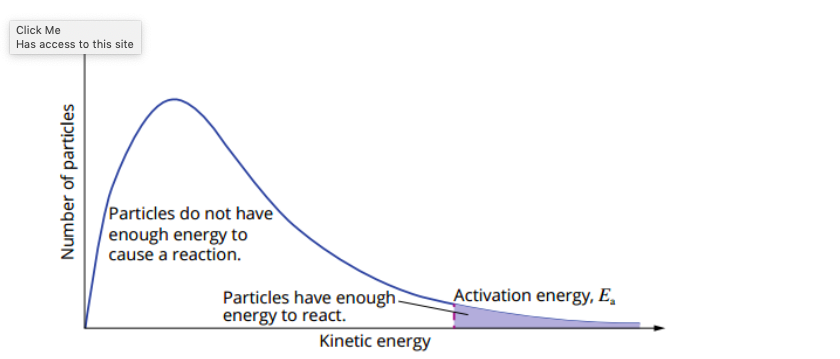
range of energies is shown on a Maxwell-Boltzmann distribution graph, aka kinetic energy distribution diagram.
what is the maxwell-boltzmann graph?
vertical axis gives the number of particles with a particular energy, while the horizontal axis gives the kinetic energies of the particles.
area under the graph represents the sum of all the reactant particles.
kinetic energy distribution diagram considers all reacting particles + their probable energy distribution,
an energy profile diagram represents the energy of individual particles during the course of a reaction.
temp and reaction rate relationship
As the temperature of a reaction system increases, the average kinetic energy of the particles increases and the average speed of the particles in the system increases as well.
describe this graph
for the range of kinetic energies for a gas at three different temperatures
area under the graph, which is equal to the total number of particles in the sample, stays constant when the temperature is changed.
As the temperature increases, the increasing average kinetic energy of the particles can be seen by the movement to the right of the peak in the Maxwell-Boltzmann graph.
what is the difference between frequency vs energy of collisions?
temperature increases, the increase in reaction rate due to the increased energy of the collisions significantly outweighs the increase in reaction rate due to the increased frequency of collisions.
give and example of the difference between frequency vs energy of collisions?
temp incr of just 10°C causes the rate of many reactions to double, but it can be shown that this is not just due to the increased frequency of collisions.
frequency of collisions only increases by about 3% when the temperature increases by 10°C.
reaction rate increases is that more particles have sufficient energy to overcome the activation energy barrier of the reaction.
does catalysts lower/increase Ea?
it lowers it.
IMPAORta