Atomic theory test
1/55
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
56 Terms
a chemical compound always contains the same elements in exactly the same proportions BY MASS
law of definite proportions
law of conservation of mass
mass cannot be created or destroyed in pedantry chemical and physical changes.
therefore, the mass of the reactants is equal to the mass of the products
when two elements combine to form two or more compounds, the mass of one element that combines with a given mass of the other is in the ratio of small whole numbers.
law of multiple proportions
john dalton
unified the ancient theory of the atom with the laws of definite proportions, conservation of mass, and multiple proportions, to come up with his eponymous atomic theory
daltons atomic theory (1)
All matter is composed of extremely small particles called atoms, which cannot be subdivided, created, or destroyed
daltons atomic theory (2)
Atoms of a given element are identical in their physical and chemical properties
daltons atomic theory (3)
Atoms of different elements differ in their physical and chemical properties
daltons atomic theory (4)
Atoms of different elements combine in simple, whole-number ratios to form compounds
daltons atomic theory (5)
In chemical reactions, atoms are combined, separated, or rearranged but never created, destroyed, or changed
JJ Thomson
Invented cathode ray tubes
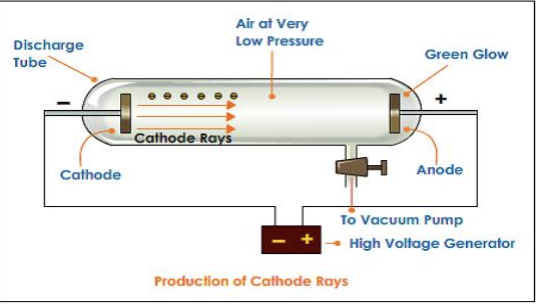
Because the cathode ray came from the — charged cathode, Thomson reasoned that the ray was negatively charged.
Thomson confirmed this prediction by seeing how electric and magnetic fields affected the cathode ray.
negatively

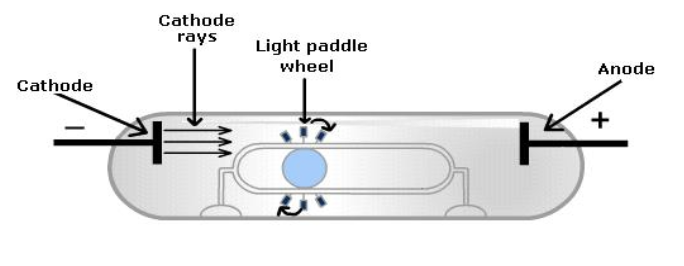
Thomson also observed that when a small paddle wheel was placed in the path of the rays, the wheel would turn.
This suggested that the cathode rays consisted of tiny particles that were hitting the paddles of the wheel.
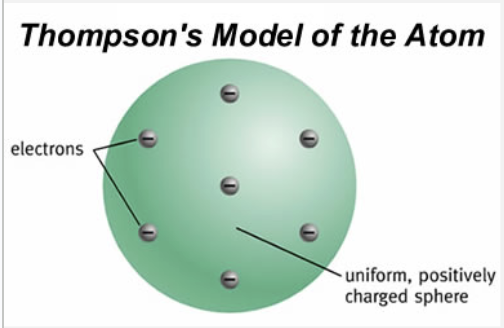
Thomson proposed that the negative electrons were embedded in a positive matrix, much like the fruit pieces in a plum pudding.
Ernest Rutherford invented the
Gold Foil Experiment
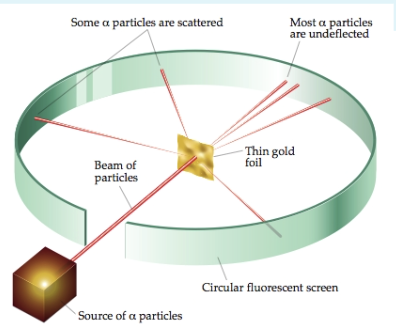
Rutherford reasoned that each atom in the gold foil contained a small, dense, positively charged nucleus surrounded by electrons. A small number of the alpha particles directed toward the foil were deflected by the tiny nucleus (red arrows). Most of the particles passed through undisturbed (black arrows).

basic arrangement of sub-atomic particles in the atom (1)
basic arrangement of sub-atomic particles in the atom
basic arrangement of sub-atomic particles in the atom (2)
The nucleus is made up of protons (positively charged) & neutrons (no charge, or neutral)
basic arrangement of sub-atomic particles in the atom (3)
The nucleus has:
- all of the positive charge
- nearly all of the mass
- only a very small fraction of the volume of the atom
basic arrangement of sub-atomic particles in the atom (4)
Electrons (negatively charged) are found outside the nucleus
Elements are defined by
the number of protons they have in their nucleus
Atomic Number =
number of Protons
Neutrons
- have no charge (are neutral)
- are found in the nucleus (with the protons)
- a neutron has the same mass as a proton
Mass Number
Mass Number equals # of Protons AND Neutrons
Isotopes
All atoms of the same element do not necessarily have the same number of neutrons; therefore their mass numbers can differ.
Atoms of the same element that have different numbers of neutrons are called isotopes
identifying isotopes

Atomic Mass Number
amu
The mass in grams of 1 mol of an element is numerically equal to
the element’s atomic mass from the periodic table in atomic mass units
The mole is the SI unit for the amount of
substance
The molar mass of an element is the mass in grams of
one mole of the element
The mass in grams of 1 mol of an element is numerically equal to
the element’s atomic mass from the periodic table in atomic mass units
avogadro's number
6.022 × 1023
An electron that is as close to the nucleus as it can be is in its
lowest energy level
The farther an electron is from the nucleus, the
higher the energy level that the electron occupies
The difference in energy between two energy levels is known as a
quantum of energy
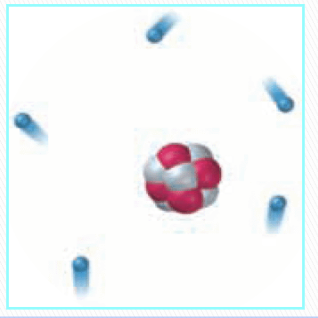
Rutherford’s model of an atom
electrons orbit the nucleus randomly (just as planets orbit the sun)
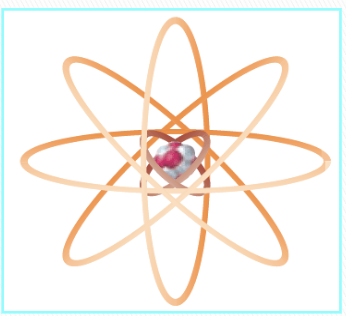
Bohr’s model of an atom
electrons travel around the nucleus in specific energy levels
As waves, electrons could have only certain frequencies which correspond to
the specific energy levels
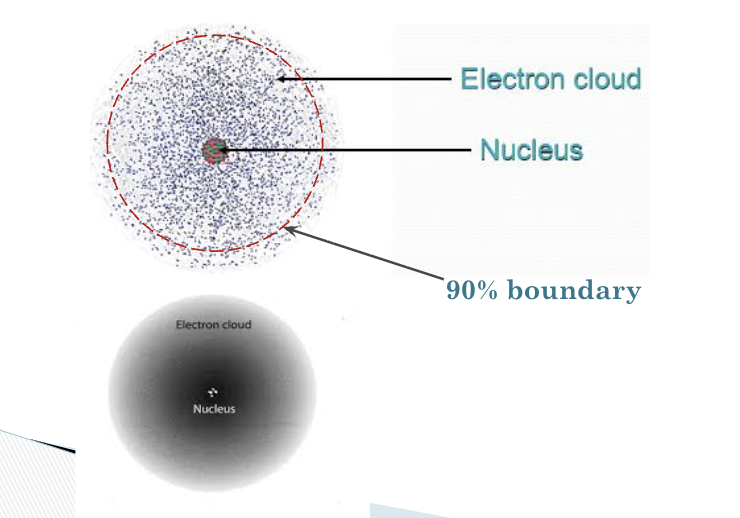
Electrons are located in orbitals, which are regions around a nucleus that correspond to
specific energy levels (where electrons are likely to be found.)
Because electrons can be in other places, orbitals have a fuzzy boundary like a cloud, and are sometimes referred to as
electron clouds
In empty space, light waves travel at
2.998 × 10^8 m/s
Electromagnetic Spectrum
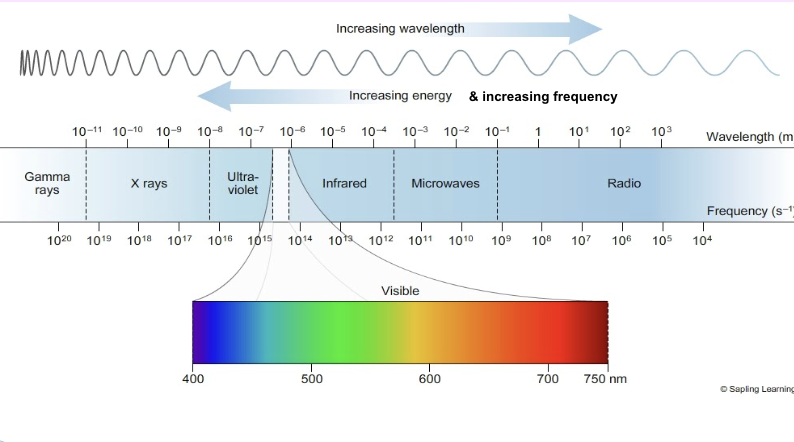
Light can be described as a stream of particles, the energy of which is determined by
the light’s frequency.
An electron can move from a low energy level to a higher energy level by
absorbing energy
Electrons at a higher energy level are unstable and can move to a lower energy level by releasing energy. This energy is released as
light that has a specific wavelength.
An electron in a state of its lowest possible energy, is defined as being
in a ground state. The ground state is the lowest energy state of an electron
If an electron gains energy, it moves to an
excited state
An excited state is a state in which an atom has more — than it does at its ground state
energy
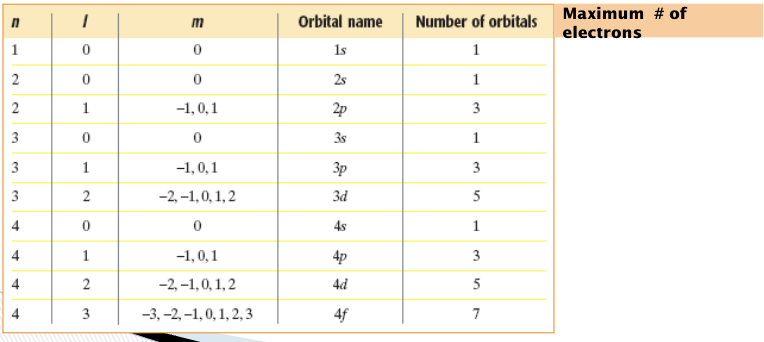
The principal quantum number, symbolized by n, indicates the main energy level occupied by the electron.
Values of n are positive integers, such as 1, 2, 3, and 4.
As n increases, the electron’s distance from the nucleus and the electron’s energy increases.
Principle Quantum Number
angular quantum number
l = 0 corresponds to an s orbital
l = 1 corresponds to a p orbital
l = 2 corresponds to a d orbital
l = 3 corresponds to an f orbital
The main energy levels (n) can be divided into sublevels ( l );
these sublevels are represented by the
angular momentum quantum number, l
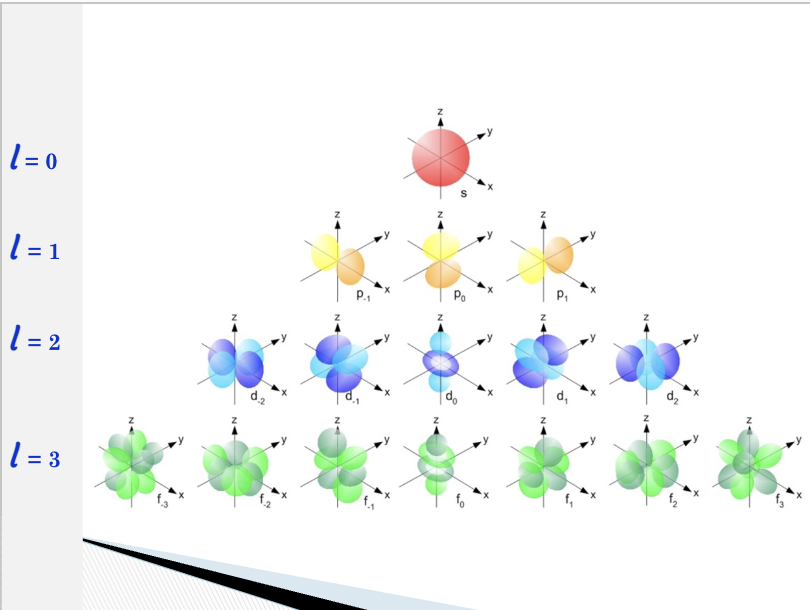
Magnetic Quantum Number (m)
One s orbital
three p orbitals
five d orbitals
seven f orbitals
The Spin Quantum Number (⬆ ⬇)
The spin quantum number is represented by
+ ½ or – ½ ( ⬆ ⬇ ) and it indicates the orientation of an electron’s magnetic field relative to an outside magnetic field.
Pauli Exclusion Principle
No more than two electrons can occupy any single s, p, d, or f orbital.
Electron configurations show the — in an atom
arrangement of electrons
aufbau principle
electrons will occupy orbitals from lowest to highest energy
hund’s rule
when there are multiple orbitals of equal energy, place one electron in each before pairing them up