chp 21 - ATP synthase
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
33 Terms
What is the chemiosmotic theory?
Proton motive force (∆ P) = Chemical Gradient (∆pH) + Electrical Gradient (∆psi)
ATP synthase uses stored energy from the eelctrochemical graident established.
No gradient → No ATP
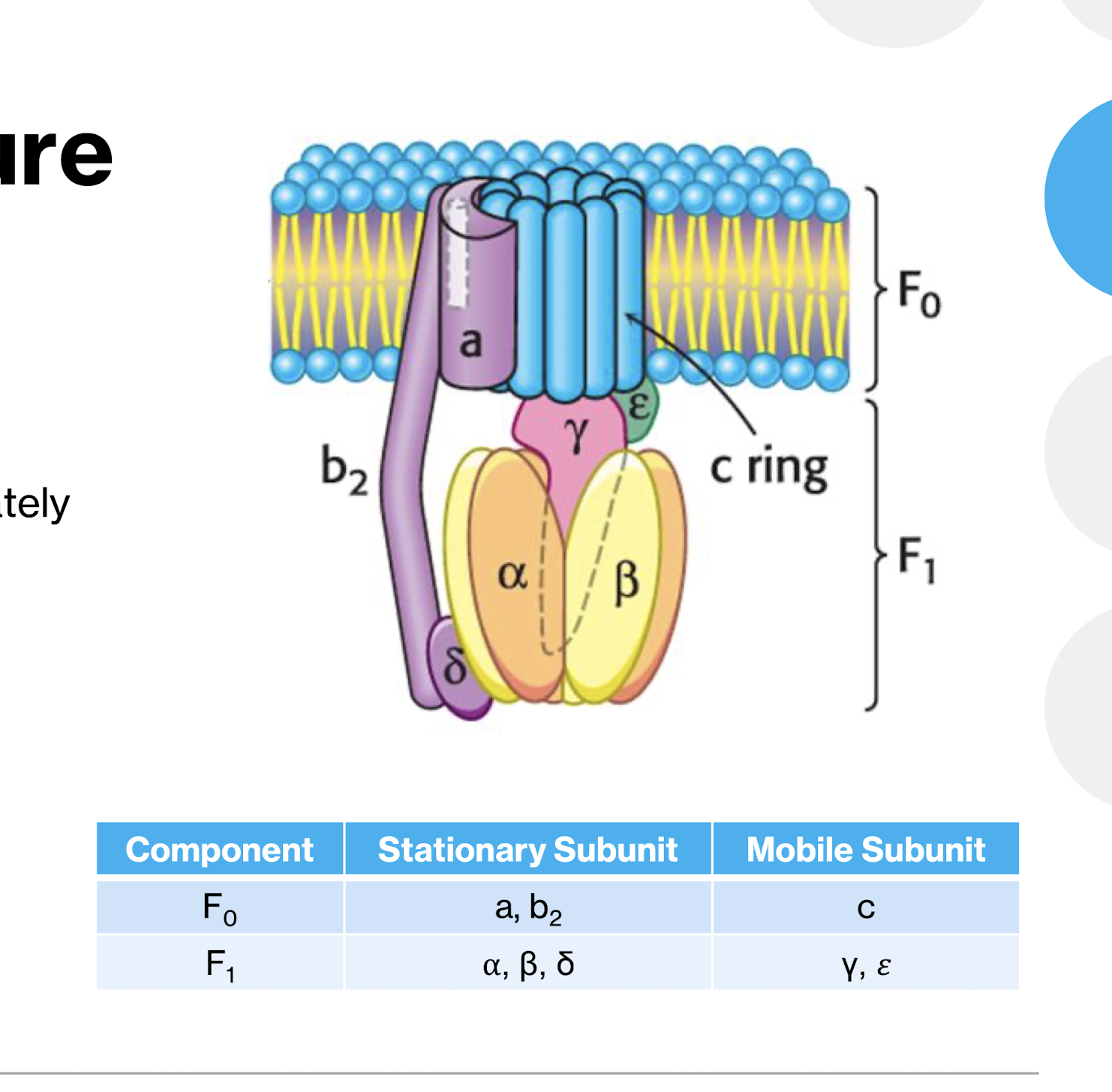
What is the structure of ATP Synthase? two main portion? Subununits of each?
F1 Portion (matric, catalytic part)
3 a subunits, 3 B subunits arranged alternating
B subunits are catalytic
Central Gamma Subunit (rotating shaft)
δ subunit (connexts F1 to F0)
ε subunit (regulatory)
F0 Portion (Membrane embedded)
c-ring (rotar)
subuit with proton channels
b2 subunit (Stator)
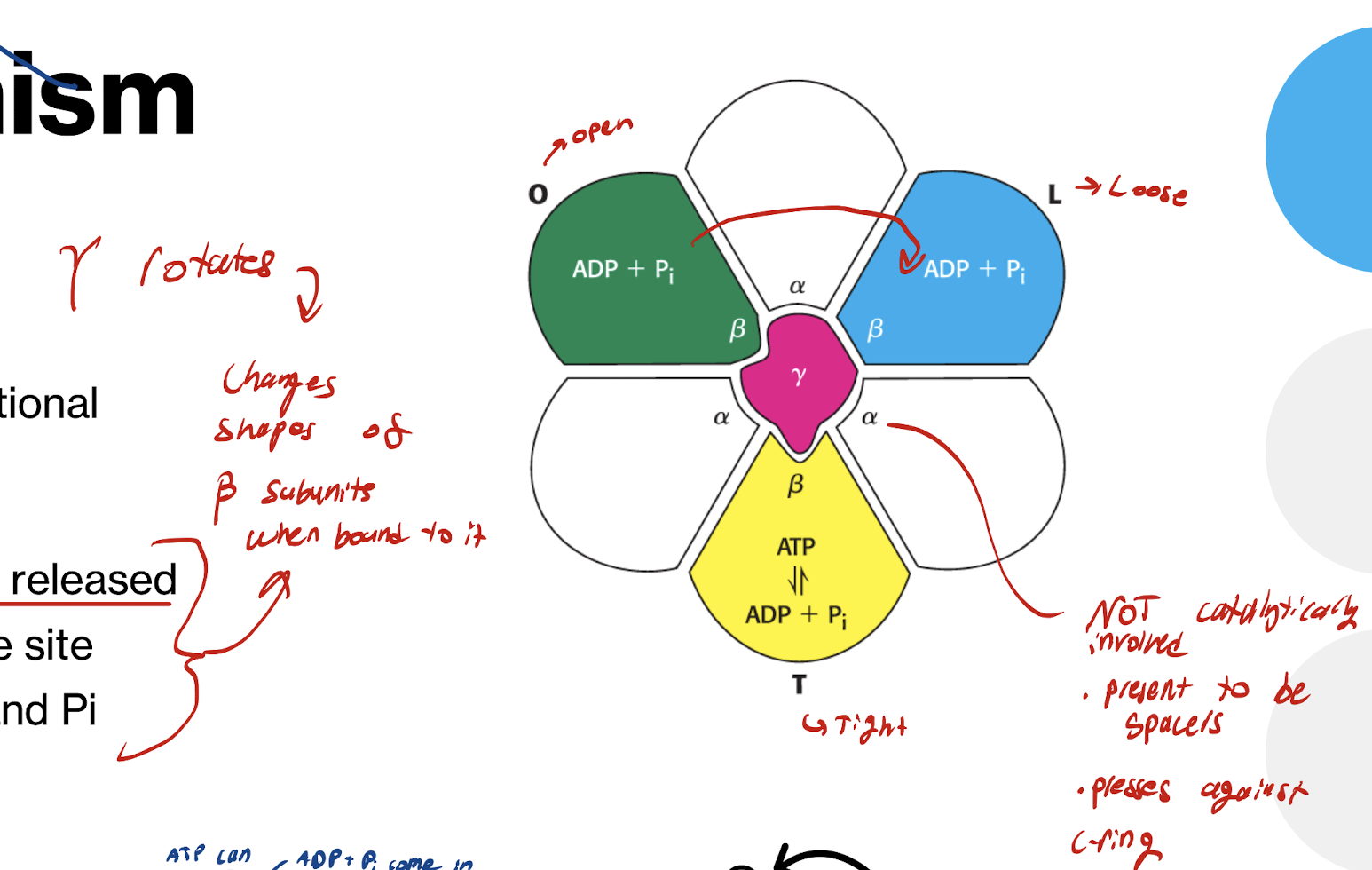
How does ATP synthase work? What is the mechanism called?
Bind-Change mechanism
What do the B subunits do? What do the a subunits do?
B → Catalytically active
a → NOT catalytically involved
Present to create space
Presses against c-ring
What moves the a and B subunits?
Gamma subunit in the middle rotates, moving a and B
a and B units themselves. DO NOT MOVE
What are the 3 conformation states of the B subunit? What happens in each? What causes the conformation change
O (open)
Nucleotides and Pi can enter
L (Loose)
Nucleotides and Pi trapped inside the active site
T (tight)
ADP and Pi compressed together forming ATP
Conformation change between subunits caused by Gamma subunit rotation
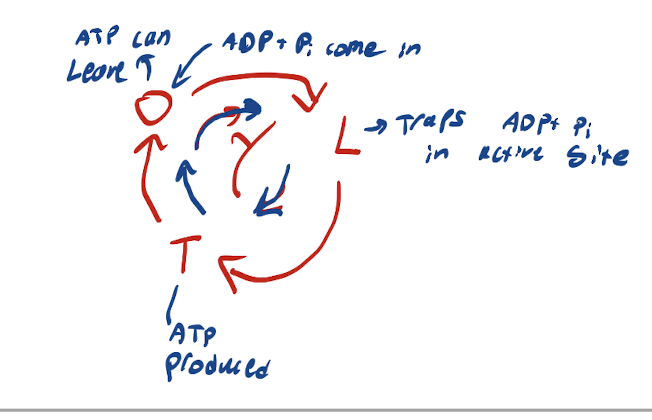
What is the cycle for the Bind-change mechanism
ADP and Pi bind to active site in O state → Gamma Rotates → ADP and Pi trapped in active site of L state → Gamma Rotates → ADP and Pi compressed to produce ATP in T state → Gamma Rotates → ATP released in O state.
What causes the Gamma subunit to rotate (what are the 4 steps)?
Proton Binding
Rotation Begins
Proton Release
Cycle Continues
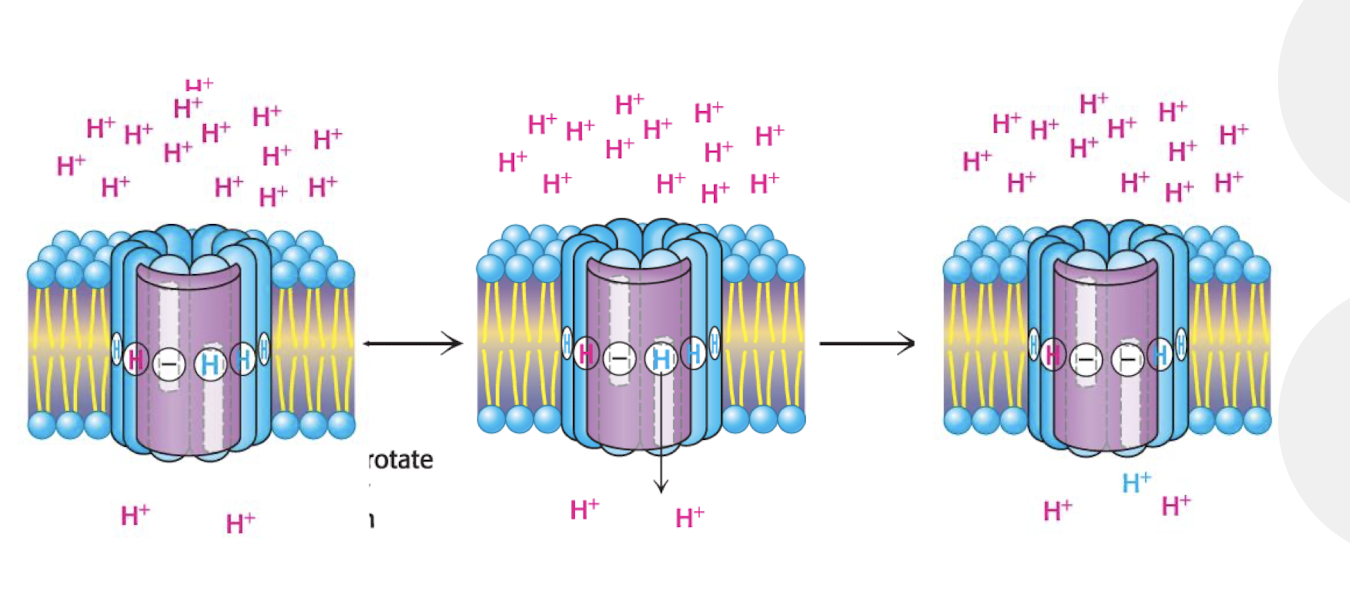
What are the ½ channels of the a subunity used for?
Two ½ channels, one at each side
one at Intermembrane side
One at matrix side
Allows H+ to enter from the Intermembrane space via IM ½ chanel, then JUMP to the matrix ½ channel via the C-ring
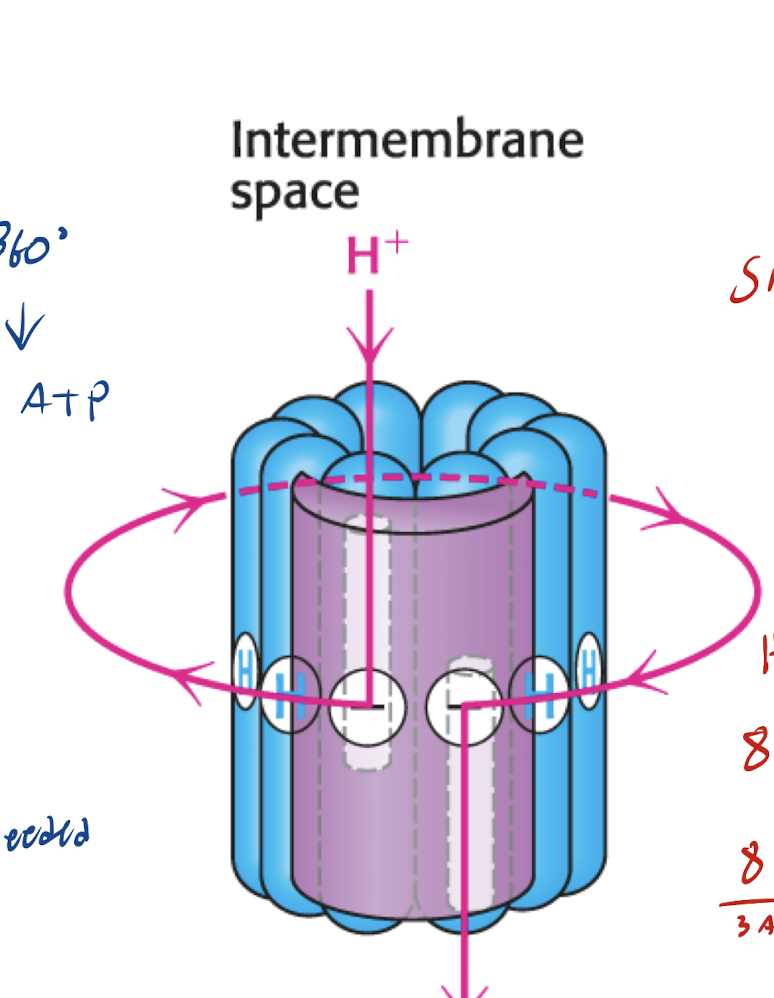
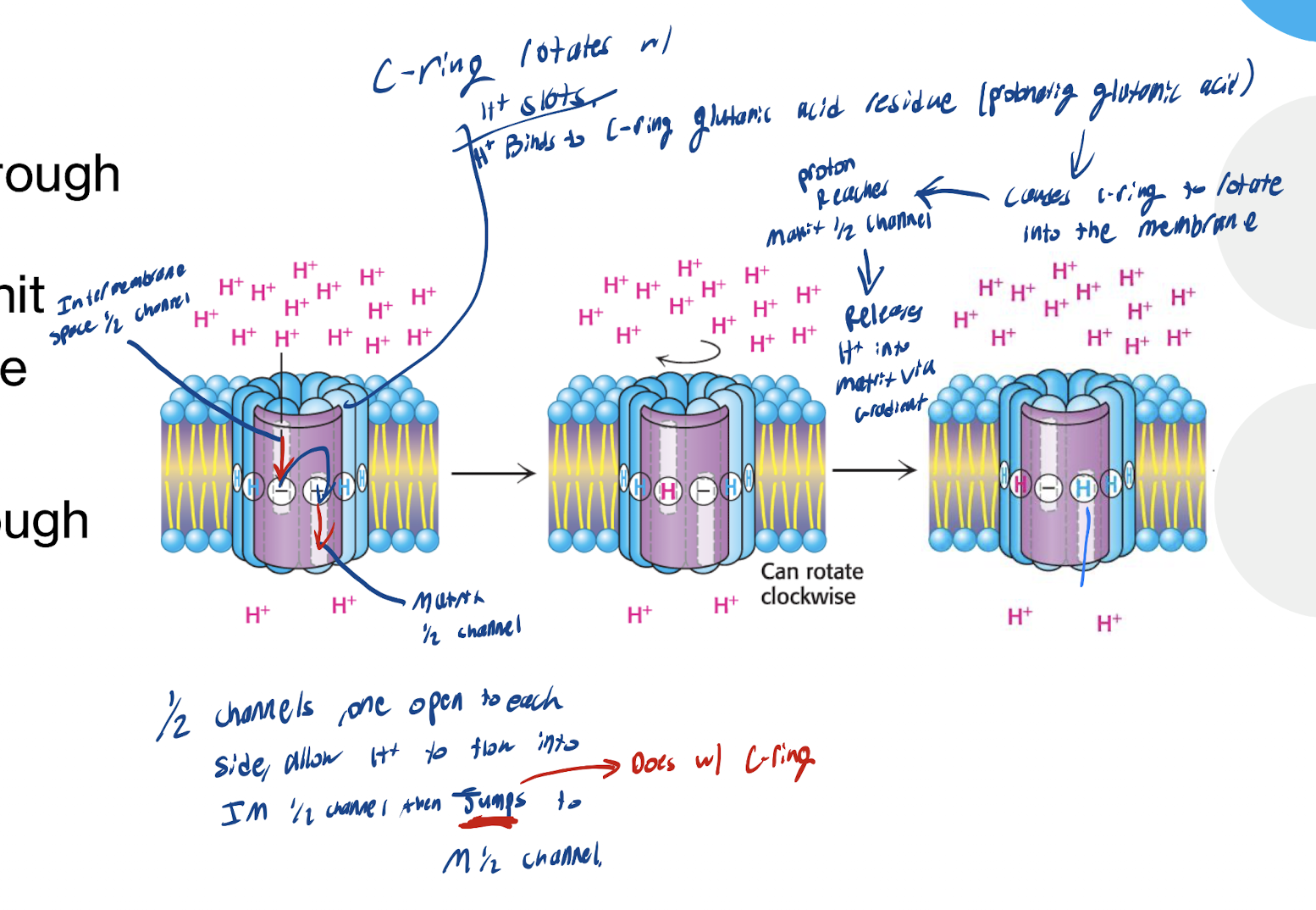
What happens in Step 1 (Proton Binding) and Step 2 (Rotation starts) of the c-ring rotation
H+ from Intermembrane Space enters the a subunit ½ channel → Protonates glutamate residue on the c subunit → Neutralizes negative charge
→ Protonated c subunit now ready to move into the lipid bilayer (b/c Hydrophobic environment favors protonated form) → c-ring rotates clockwise with the H+ still at the end of the IM ½ Channel.
What happens in Step 3 (Proton release) and Step 4 (cycle continue)
Step 3 - Proton Release
Protonated c subinit reaches the matrix facing ½ channel (since all the other ½ channels of the a subint have H on them, and each are waiting their turn to arrive to the matrix facing ½ channel) → high pH of matrix favors deprotonation → H+ in the Matrix ½ channel is released into the matrix via the ½ channel.
Step 4 - Cycle continues
The Deprotonated c subunit rotates again → The next H+ will come in and bind repeating the process
Continous rotation is driven by proton flow
Sample problem
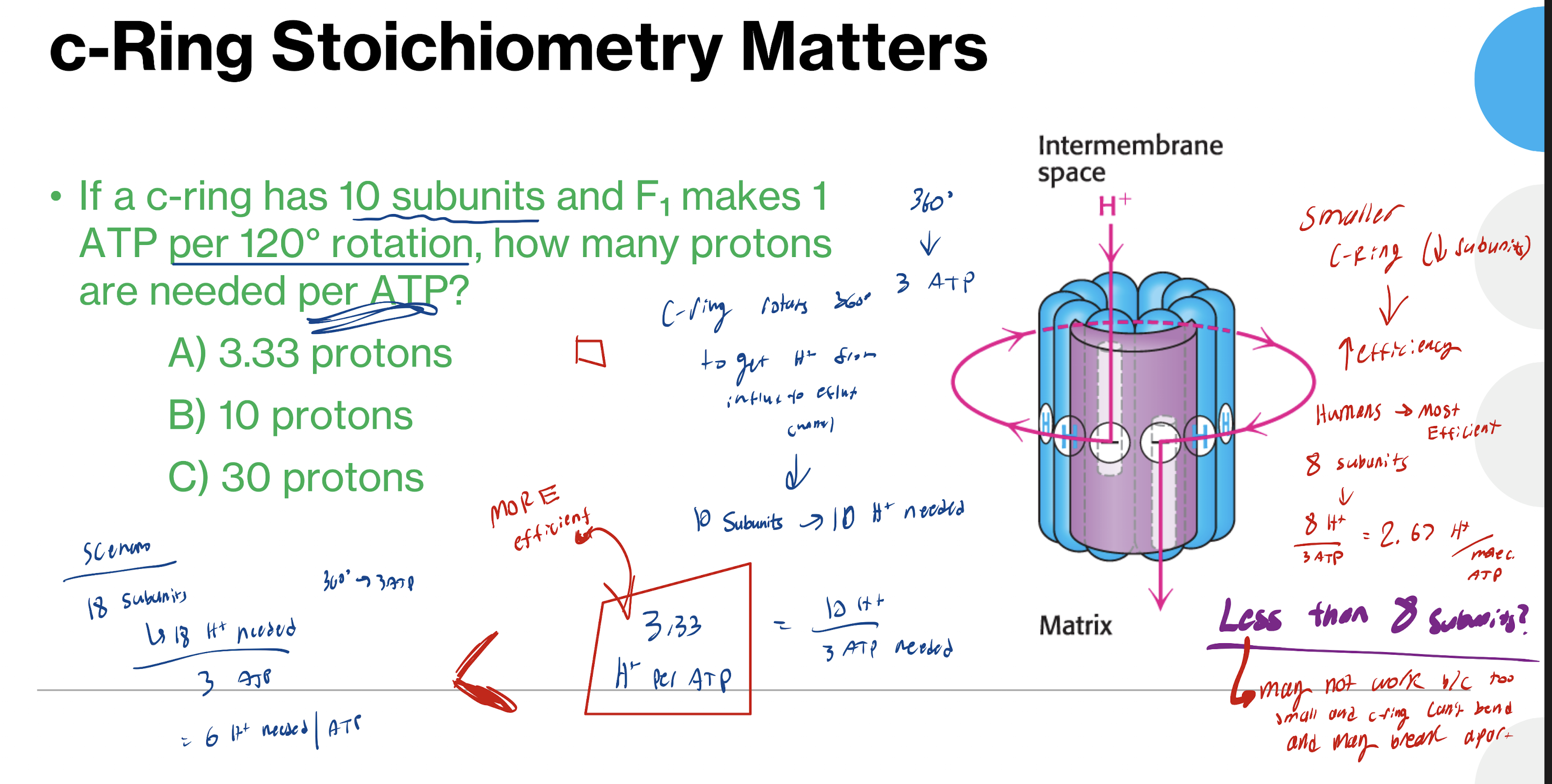
How do you calculate how many protons are needed per ATP with c-rings having a specific number of subunits
Number of subunits = number of H+ needed
#H+ needed/#ATP produced from rotation
Ex: 1 ATP every 120o rotation → 3 ATP
Are smaller or bigger C-rings more efficient at making ATP (less H+ needed to make 1 ATP)? Who is most efficient?
Smaller C ring (less subunits) → INC efficiency
humans → 8 subunits
2.67 H+ needed per ATP
8 is the lowest number of subunits possible
any smaller causes structural problems
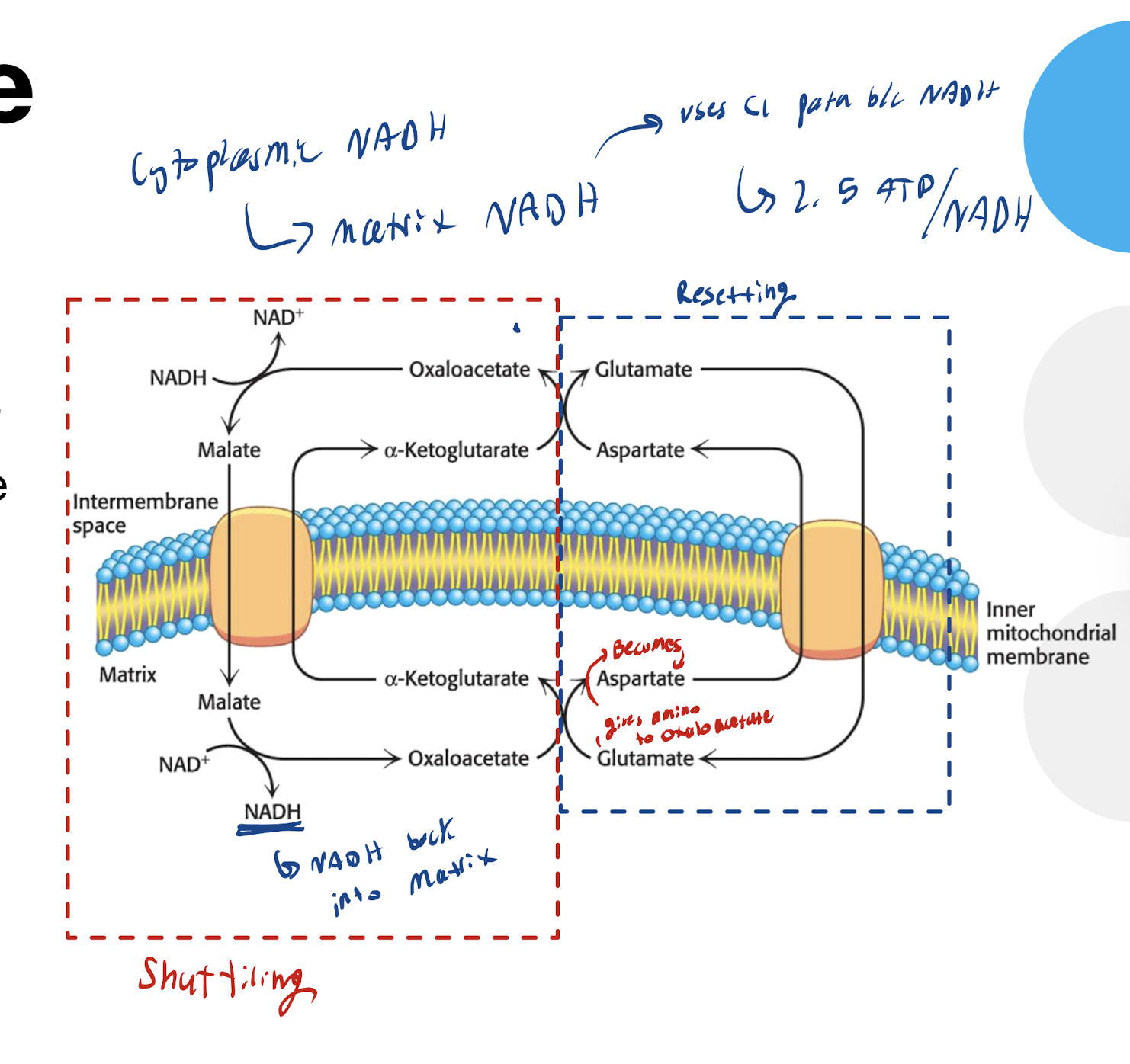
What do cytosolic NADH shuttles do? Why are they needed?
Allow NADH to travel throuhg the inner mitochondrial membrane (since it can’t do by itself)
Allow more energy extraction
What are the two types of shuttles used. Why are two different ones used?
GLycerol 3-Phosphatate Shuttle
Malate-Aspartate Shuttle
Different ones used because they are needed for diff functions by diff body tissues
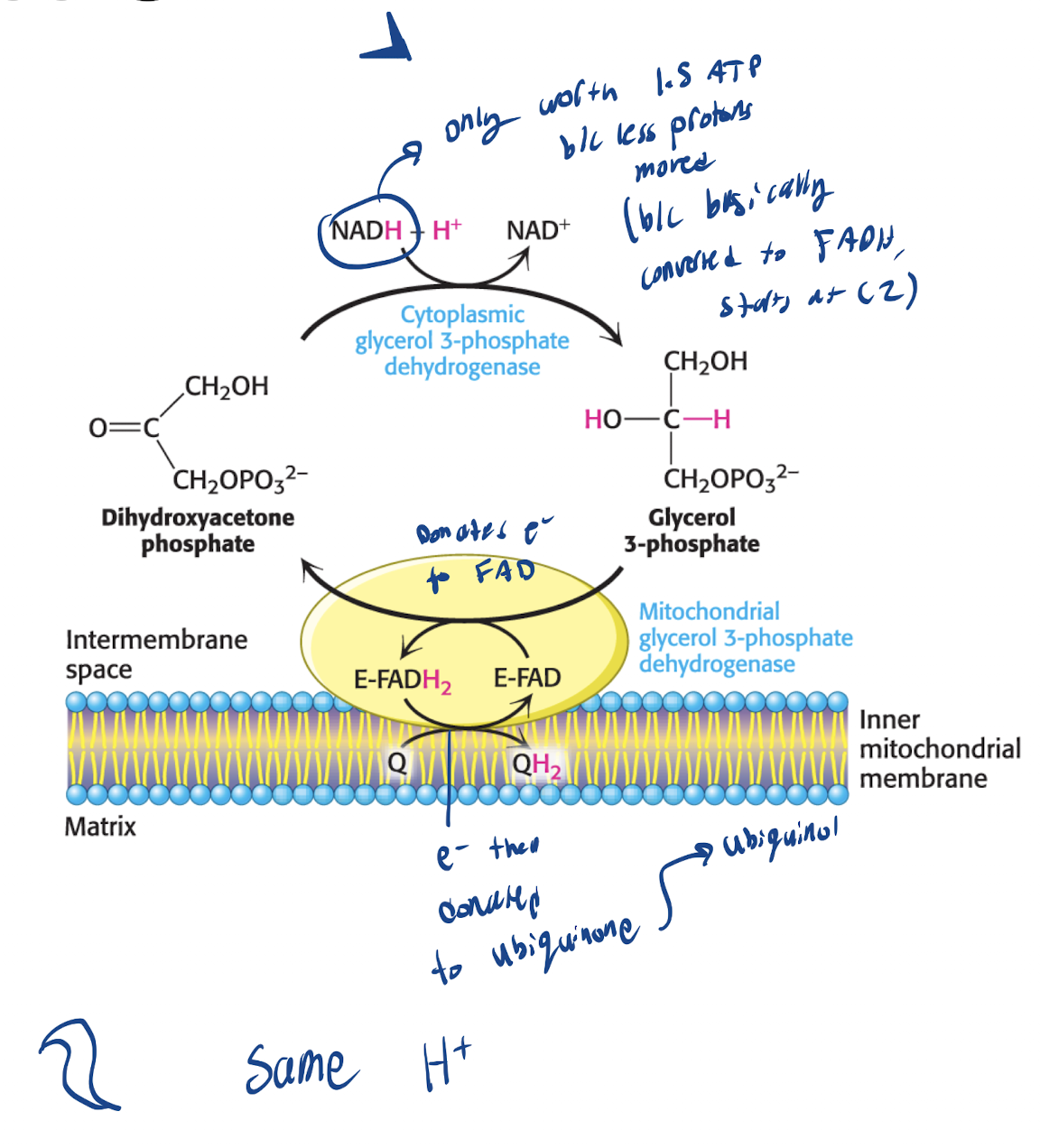
Where is the Glycerol 3-Phosphate shuttle used? What’s an advantage and trade off?
High energy tissues (Brain, skeletal muscle)
Advantage: Simple, fast mechanism for high energy tissues. Used in emergencies (need ATP now)
Trade-off: Low ATP yield b/c uses FADH2 instead of NADH
What is the mechanism of the Glycerol 3-phosphate pump?
Cytoplasmic NADH used to reduce DAP —Glycerol 3-phosphate Dehydrogenase→ Glycerol 3-Phosphate
Glycerol 3-phosphate crosses outer membrane of Mitochondria → Gets oxidized by mitochondrial Enzyme as FAD reduced to FADH2 → Electrons passed to QH2 and enters complex 3 (bypassing complex 1)
Where is the Malate-Aspartate Shuttle used? Advantage and disadvanatge?
Used in heart and liver (want a lot of ATP to produced)
ADvantage: Maintatins NADH in matrix → Full yield obtained via Complex 1
Disadvantage → More complex mechanism → Needs more transporters and enzymes
What is the simplified mechanism of the Malate-Aspartate Shuttle
Cytoplasmic NADH reduces Oxaloacetate into Malate → Malate enters mitochondria via malate-a-ketoglutarate antiporter → Malate now in matrix → Malate formed back to oxaloacetate via malate dehydrogenase, producing NADH (which is then used to trasnfer electrons starting at C1)
Transamination rxn regenerates transportable forms
What does ADP/ATP translocase do? What is it powered by?
Transports ATP out of the mitochondria to be used and ADP into the mitochondria so ATP can be produced
Powered by proton motive force?
How does the ADP/ATP translocase work? What is essential for the transport
ADP enters the translocase on ECF side → Conformation change → ADP exits into the mitocondria (now open) → ATP enters the translocase (attracted to binding site via electrical gradient aka positive charge of intermembrane space) → conformaation change → ATP released to oustside
Only able to happen because of the proton gradient (allows ATP to enter into the translocase)
No gradient → No ATP transport
What is the complete ATP yield from Glucose oxidation assuming malate aspartate shuttle is used? How much for substrate and oxidative

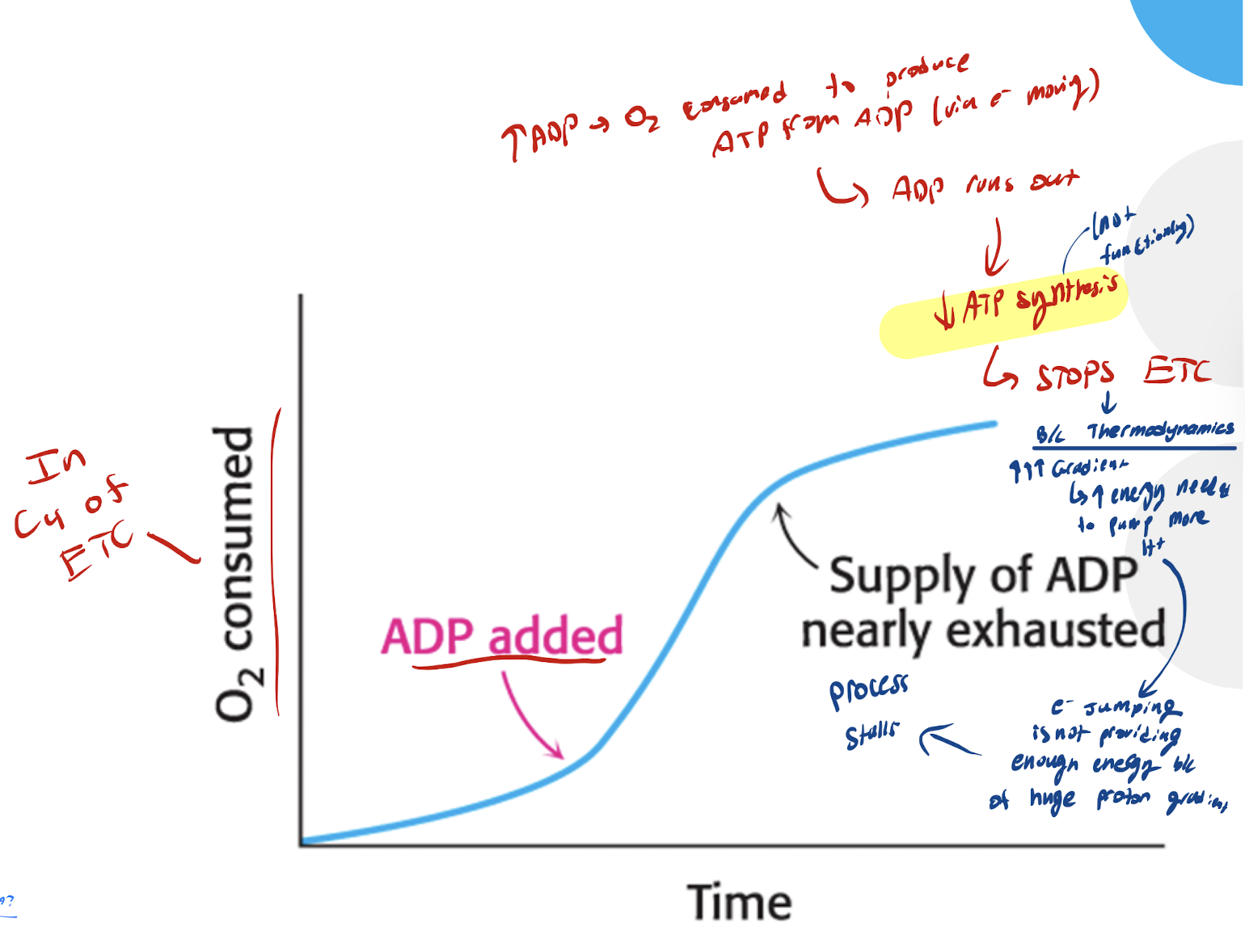
What happens iwth ATP synthesis if there’s no ADP?
No ADP → No ATP SYnthase (because no ADP substrate present for the ATP synthase to use)
Causes proton fgradient to build up too much and inhibits electron transport
What is the mechanism of ATP synthesis decreasing when ADP supply runs out?
ADP present → O2 consumed to produce ATP from ADP → ADP runs out → Dec ATP synthesis → STOPS ETC (b/c thermodynamics. INC INC INC gradient built up → INC energy needed to pump H+ out) → e- jumping bweteeeb protons not providing enough energy because of. thehuge gradient → The process stops
When there is little ADP, What happens with the TCA, PDH complex, and Glycolysis? Why?
System is tihgly coupled

What is a natural example of uncoupling?
Brown ADipose Tissue
What protein is involved with brown adipose tissue? What is the mechanism and result?
UCP-1 (thermogenin) → Protons bypass ATP synthase in Inner mitochondrial membrane → Alternative is used as bypass → DEC proton gradieint → INC ETC to fill that H+ Gradient → Energy then released as heat instead of capturing ATP → Non-shivering Thermogenesis
What uses non--shivering thermogenesis? What can’t use?
Hibernating animals and newborns use
Pigs can’t use bc they don’t have the uncopling protein
What do Induecd Uncouplers (DNP and UCP) do?
DNP lipid-soluble inserting into the IM
Dissipate proton gradient
ETC continues, no ATP made
Ferries protons across inner mitochondrial membrane
Heat generated
How is DNP historically used?
was marketted as weight-loss drug, but banned because of hyperthermia reports (because of the releasing of heat)
How was DNP toxic?
Uncoupling action is unregulated and was happening in each tissue. Slight overdoses completely change body temp and can cause tachycardia and acidosis
What do induced inhibitors do (specifically ATP synthase inhibitors like oligomycin)
Builds up the proton gradient
Blocks flow through the F0 subunit of ATP synthase (causes gradient to build up)
ETC also then stops
because the gradient is so strong that the energy released from the electrons moving down the ETC is not enough to pump out H+, so it stalls