Chemistry EOY
1/75
Earn XP
Description and Tags
Metals, chemical tests, halogens and the atmosphere
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
76 Terms
Alkali metal group
Found in group 1 as they only have 1 electron in their outermost shell
Physical properties of alkali metals
Good conductors of heat and electricity
Soft compared to other metals
Low density compared to other metals
Low melting/boiling points compared to other metals
Chemical properties of alkali metals
Form ionic compounds with non-metals
React violently with chlorine
Burst into flames when heated with oxygen
Produce soluble white compounds
React with cold water
Pattern of reactivity in alkali metals as you go down
As outer electrons get further from the nucleus, there is less force of attraction making the electron more likely to bond with another atom, increasing reactivity
Patterns in alkali metals (aside from reactivity) as you go down
Melting/boiling point decrease
Density increases
Softness increases
General equation for alkali metal reaction to water
Metal + water —> metal hydroxide + hydrogen
Observation of potassium and cold water + equation
Reacts very violently; explodes as it glides across the water
Hydrogen gas is produced and ignited by heat
Water becomes alkali
2K₍ₛ₎ + 2H₂O₍ₗ₎ —>2KOH₍ₐq₎ + H₂₍₉₎
Observation for sodium and cold water + balanced equation
Reacts violently, gliding across the water
Hydrogen gas produced and MAY ignite and explode
Water becomes alkali
2N + 2H₂O —>2NaOH + H₂
General equation: group 1 metal with chlorine
group 1 metal + chlorine —>metal chloride
Balanced equation for lithium and chlorine
2Li +Cl₂—>2LiCl
General equation: metal and heated oxygen (fire)
Metal + oxygen—> metal oxide
Balanced equation for potassium and oxygen
2K + O₂—>K₂O₂
Observation of magnesium and oxygen (fire)
Starts silver shiny metal
Bright white light of sparks during reaction
Produces white powder of MgO
Observation of iron and oxygen (fire)
Starts a dull and grey metal
Iron glows hot, sparks, during reaction
Produces rust-coloured FeO
General equation: metal with hydrochloric acid (HCl)
metal + hydrochloric acid—>salt + hydrogen gas
Observation of copper with HCl
No reaction at all
Observation of iron with HCl
Very slow fizzing
Hydrogen present (squeaky pop)
Observation of zinc with HCl
Slow fizzing, few bubbles
Hydrogen present (squeaky pop)
Observation of magnesium with HCl
Exothermic reaction
Fizzing
Hydrogen produced (squeaky pop)
Displacement reactions
A more reactive metal will displace a less reactive metal from it’s aqueous solution of one of its salts
Displacement reactions of magnesium with metal sulphates
Displaces CuSO₄ (black powder formed), ZnSO₄ (turned black), FeSO₄ (turned slightly black and dissolves slightly)
The most reactive
Displacement reactions of copper with metal sulphates
Displaces no metal sulphates
The least reactive
Displacement reactions of zinc with metal sulphates
Displaces CuSO₄ (turned black), FeSO₄ (solution turns colourless)
The second most reactive
Displacement reactions of iron with metal sulphates
Displaces CuSO₄ (rust formed)
The third most reactive
Define an anion
An atom that has gained an extra electron and thus becomes more negatively charged
Define a cation
An atom that has lost an electron and thus becomes positively charged
Method for flame test
Dip platinum loop into solid sample and place on the edge of blue flames (must be a metal that has low reactivity and high melting point)
Record result
Clean loop with HCl
Repeated process
Flame test for cations: Li⁺
Flame becomes brick-red
Flame test for cations: Na⁺
Flame becomes yellow
Flame test for cations: K⁺
Flame turns lilac
Flame test for cations: Ca²⁺
Flame becomes orange-red
Flame test for cations: Cu²⁺
Flame turns green-blue
Sodium hydroxide test for cations method
Add NaOH (sodium hydroxide) into the solution
Record result (typically precipitate)
Sodium hydroxide test for cations: Cu²⁺
Blue precipitate of Cu(OH)₂ is formed
Sodium hydroxide test for cations: Fe²⁺
Green precipitate of Fe(OH)₂ is formed
Sodium hydroxide test for cations: Fe³⁺
Brown precipitate of Fe(OH)₃ is formed
Test for ammonium ions (NH⁴⁺) method
Add sodium hydroxide (NaOH) to ammonium chloride
Warm gently with Bunsen burner
Test gas produced using damp, red litmus paper
If ammonia gas is present, red litmus paper turns blue
Equation for ammonium chloride and sodium hydroxide (symbol)
NH₄Cl + NaOH—>NaCl +NH₃ + H₂O
Equation for ammonium chloride and sodium hydroxide (word)
Ammonium chloride + sodium hydroxide—>sodium chloride + ammonia gas + water
Method for testing halide ions
Remove carbonate ions with dilute nitric acid
Add silver nitrate (AgNO₃)
Observe precipitate formed
Test for halide ions: iodide (I⁻)
Yellow precipitate of AgI formed
Test for halide ions: bromide (Br⁻)
Cream precipitate of AgBr formed
Test for halide ions: chloride (Cl⁻)
White precipitate of AgCl formed
Method for testing sulphate ions (SO₄²-)
Add hydrochloric acid to remove carbonate ions
Add barium chloride (BaCl₂) to the solution
Sulphate ions present—>white precipitate formed
Method for testing carbonate ions (CO₃²⁻)
Add hydrochloric acid (HCl) to solution; if effervescence present, test for CO₂
To test for CO₂, run the gas through lime water
Carbonate ion—>solution turns cloudy/milky
Test for carbon dioxide (CO₂)
CO₂ is a colourless, odourless gas
If you bubble it through limewater and it turns cloudy/milky, CO₂ is present
Test for hydrogen (H₂)
Hydrogen is a colourless, odourless gas
When you place a lit splint near the mouth of the test tube and it makes a squeaky pop sound (small explosion), hydrogen is present
Testing for oxygen (O₂)
Oxygen is a colourless and odourless gas
By placing a glowing splint (barely lit) at the mouth of a test tube and it lights back up, oxygen is present
Oxygen is needed to light fire
Testing for chlorine (Cl₂)
Chlorine gas has a pale green colour and a choking smell
Place damp blue litmus paper near the mouth of a test tube and if the paper turns white, chlorine gas is present
When the litmus paper turns white, we call the paper “bleached”
Test for ammonia gas
Ammonia is a colourless gas with a pungent smell
Place damp, red litmus paper near the mouth of the test tube and if the litmus paper turns blue, ammonia gas is present
Test for water (chemical way)
Pure copper sulphate is white when there is no water
When copper (II) sulphate turns blue when the solution is poured on it, water is present
Define halogens
Elements in group 7 that have seven electrons in their outer shell. They are non-metals and consist of diatomic molecules
Physical observation of Fluorine (F)
Gas at room temperature
Yellow in colour
Physical observation of chlorine (Cl)
Gas at room temperature
Pale green in colour
Green-blue in solution
Physical observation of bromine (Br)
Liquid at room temperature
Red-brown in colour
Orange in solution
Physical observation of iodine (I)
Solid at room temperature
Black in colour
Dark brown in solution
Patterns of halogens as you go down
Melting/boiling point increases
Colour becomes darker
Reactivity decreases
Reactivity of halogens
As you go down group 7, the halogens become less reactive. This is because as the outer shell grows further away from the nucleus, it is harder to attract another electron
Gases in the atmosphere
Nitrogen 78%
Oxygen 21%
Argon 0.9%
Carbon dioxide 0.04%
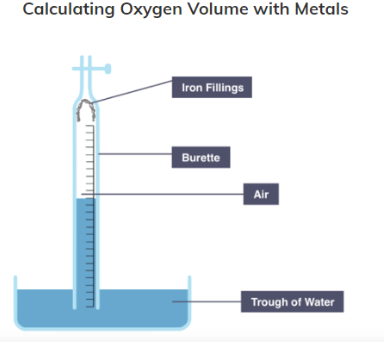
Method for calculating oxygen using iron
Place iron fillings at the end of a burette
Use clamp to hold burette vertically in trough of water
Measure the initial height of water in the burette
Leave apparatus for weeks
Measure and note the final height of water level in the burette
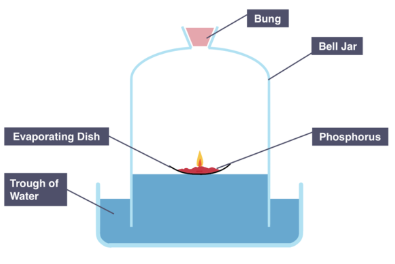
Method of calculating oxygen using phosphorous
Add Phosphorus onto an evaporating dish and place it on a trough of water, making sure it is floating
Ignite Phosphorus using a candle
Cover this with a bell jar
Measure and note the starting height of the water level in the bell jar
Leave apparatus for several days
Measure and note the final height of the water level in the bell jar
Calculating the amount of oxygen used in rusting
Volume of air at start = total burette volume - initial burette reading
Volume of oxygen used = initial reading - final reading
Percentage of oxygen = (volume of oxygen used ÷ volume of air at start) × 100
Define combustion
A chemical reaction between a substance and oxygen, producing an exothermic reaction with the substance, also known as burning
Observation of combusting magnesium
Intense white flame
White powder produced (magnesium oxide)
2Mg + O₂—>
Observation of combusting hydrogen
Exothermic
Water is produced
2H₂ + O₂—>2H₂O
Observation of combusting sulfur
Blue flame
Forms colourless, poisonous gas (sulphur dioxide: can lead to acid rain)
S + O₂—>SO₂
Define thermal decomposition
The process in which heat (thermal energy) is being used to break chemical substances down
General equation for thermal decomposition
Metal carbonate—>metal oxide + carbon dioxide
Symbol equation for copper thermal decomposition
CuCO₃—>CuO + CO₂
The difference between global warming and climate change
Global warming is the result of human activities warming the earth; it is the cause of climate change
Climate change is the effect of global warming including change of weather, winds, etc.
Effect of greenhouse gases
Greenhouse gases absorbs infrared radiation (sun rays and warms up the atmosphere. At regular amounts, they are essential for life but if the concentration is too high, it leads to global warming.
Examples of greenhouse gases
Methane
Nitrous oxide
Chlorofluorocarbons
Carbon dioxide
Problems resulting from global warming
Ice sheets melting—>sea levels rise, increase flood risk for coastal countries
Warmer atmosphere—>extreme weather
Change in climate—>crops and animals are harder to raise
Increase in smog—>heart disease, lung cancer, asthma
Define rusting
When iron/steel corrodes. The rust is essentially just hydrated iron oxide (see equations below)
Iron + oxygen + water—>hydrated iron (III) oxide
4Fe + 3O₂ + 𝑥H₂O—>2Fe₂O₃ • 𝑥H₂O
Rust prevention: barrier
By coating iron with a barrier, it prevents iron from coming into contact of water and oxygen, avoiding rust
Common barrier methods include paint, oil, grease, and plastic
However, if the coating is washed away, the iron will rust
Rust prevention: galvanising/sacrificial
Iron is coated in a layer of zinc, ZnCO₃
Since zinc is more reactive than iron, it will react with the oxygen before it can reach the iron, thus preventing rust
If the coating of zinc is scratched, iron is still protected from rusting by the sacrificial method