Exam 4 heme lecture
1/65
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
66 Terms
Anemia sources of error
Hypervolemia= overhydration leads to HCT and HGB falsely decreased
Hypovolemia= Dehydration leads to HCT and HGB falsely elevated
Acute blood loss= Plasma and RBCs are lost equally so may appear normal
- plasma is replaced first causing HGB and HCT to be decreased while recovering
Bohr Effect
Aerobic glycolysis causes lactic acid buildup making HGB O2 affinity decrease due to acidosis
- causes increased O2 in tissues
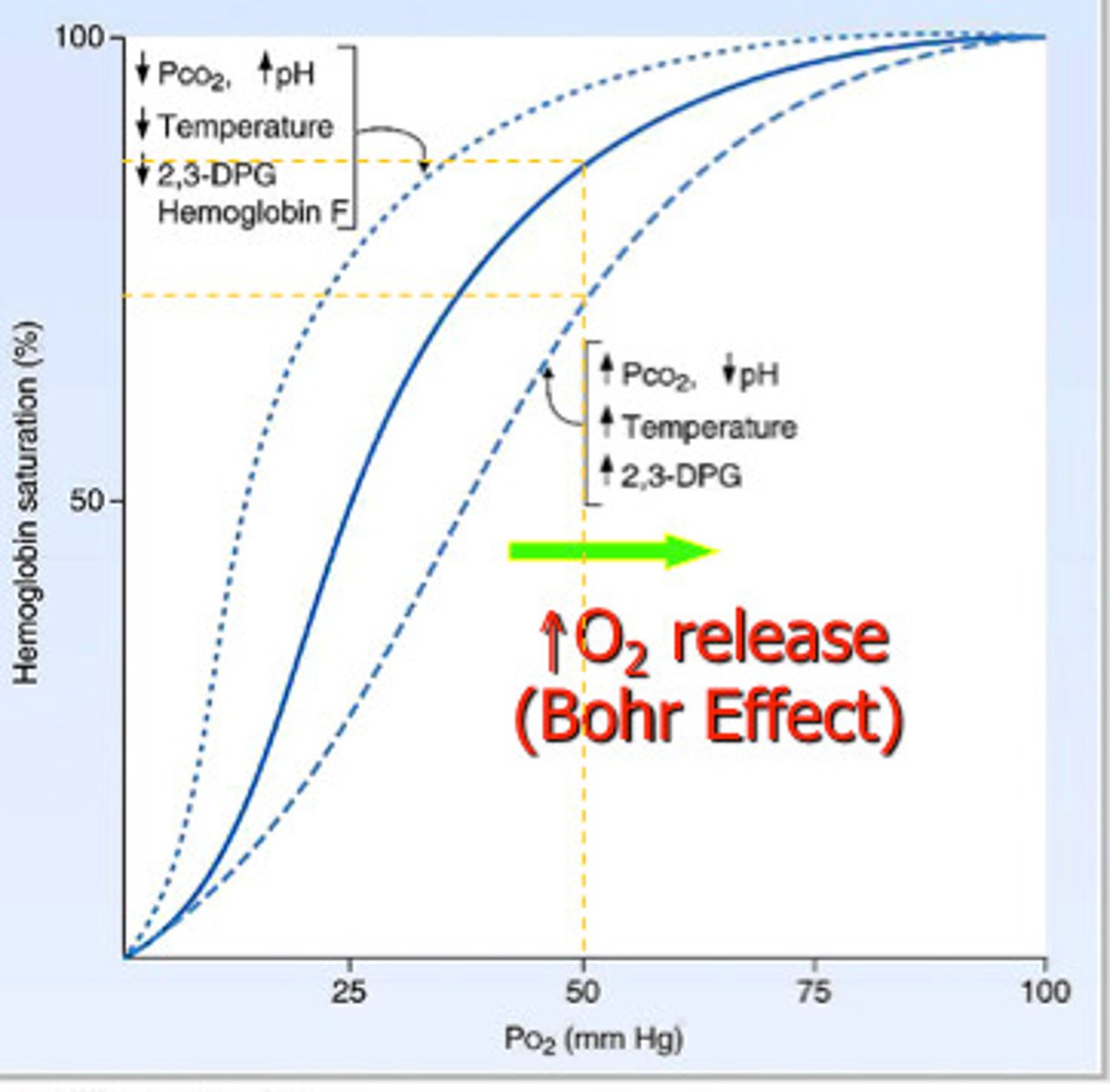
Factors shifting O2-HGB Disassociation curve to the right
Bohr effect, decreased pH, increased temp, increased CO2, increased 2,3 BPG
Normal RBC kinetics equation
M= P * S
M= amount of mass of RBCs (mL)
P= number of RBCs produced (mL/day)
S= Length of RBC survival (days)
Moderate anemia
HGB is 7-10 g/dL
Severe anemia
HGB is <7 g/dL HGB
Functional anemia
by mechanism, either less/ destruction of RBCs or impaired production
Ineffective erythropoiesis
Defective RBC precursors (maturation, survival, or proliferative defect) - B12/folate deficiency, thalassemia, etc
Morphological anemia
Based on indices and reticulocyte count
Insufficient Erythropoesis
decreased number of RBC precursors causing decreased production
- iron deficiency, EPO deficiency, autoimmune, or BM suppression (leukemia)
Normal adult relative reticulocyte
0.5 - 2.5%
Normal absolute reticulocyte count
20-115 * 10^3 / uL
Corrected Reticulocyte count
(patient HCT/ Normal HCT) * % reticulocytes
normal= 45% male or 42% females

Reticulocyte production index (RPI)
corrected reticulocyte count %/ expected maturation time
>3 = hemolysis
>2= effective erythropoiesis
<2= ineffective erythropoiesis
Functional anemia classifications
Proliferative defects= BM damage, tissue infiltration, HSC issues
Maturation defects= nuclear or cytoplasmic defects
Survival defects= hemolysis or hemorrhage
Normocytic, normochromic
if increased reticulocytes then the body is recovering from something: acute hemorrhage, hemolytic anemia, etc
if decreased or normal reticulocyte count then body is not responding properly and greater problem: Renal insufficency or failure, BM cancer, combined anemias, etc
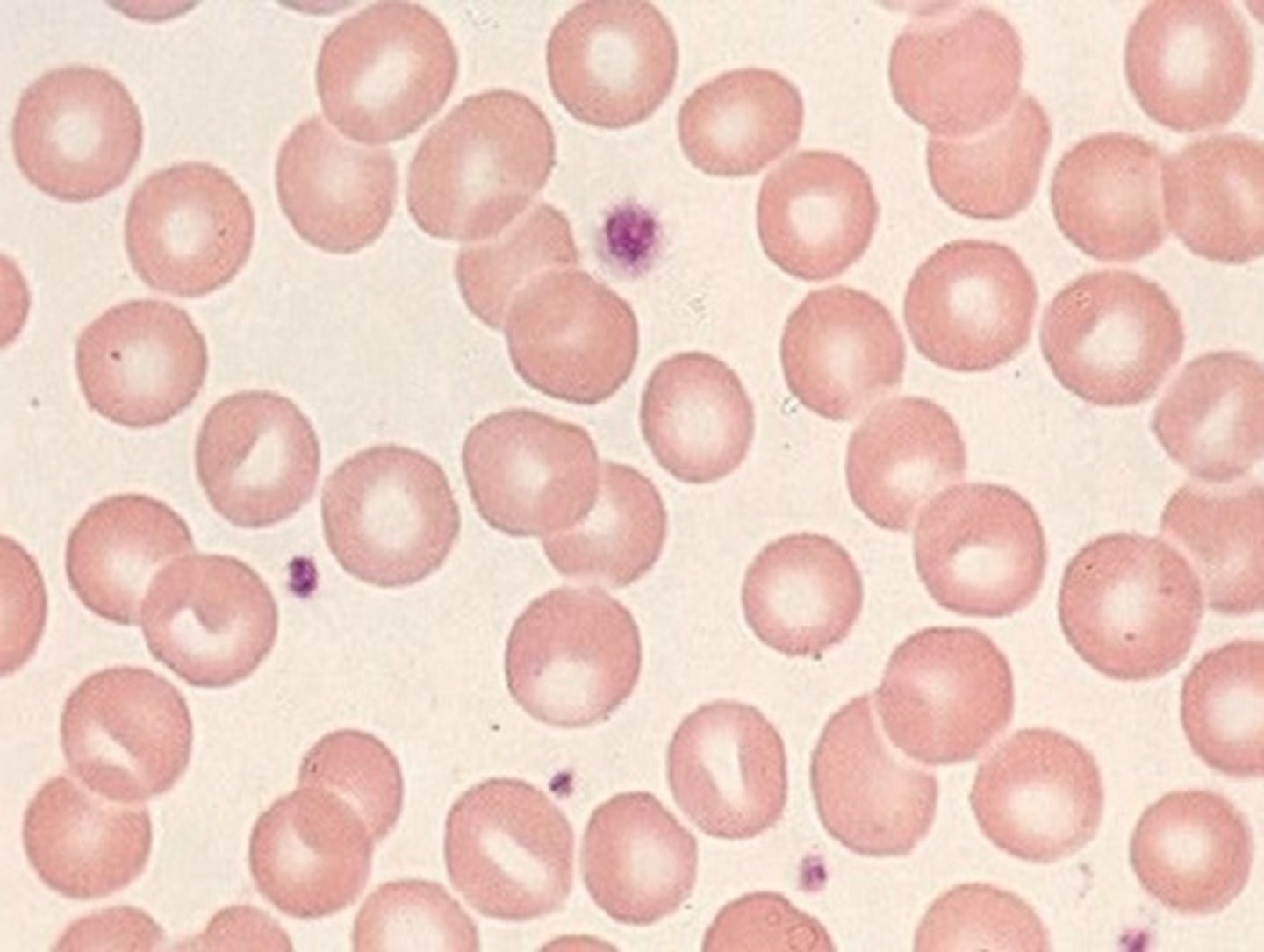
Microcytic
If decreased ferritin then iron deficency
if increased or normal ferritin then thalassemia, sideroblastic anemia, or anemia of chronic disease
Thalassemia
inherited blood disorder with decreased HGB
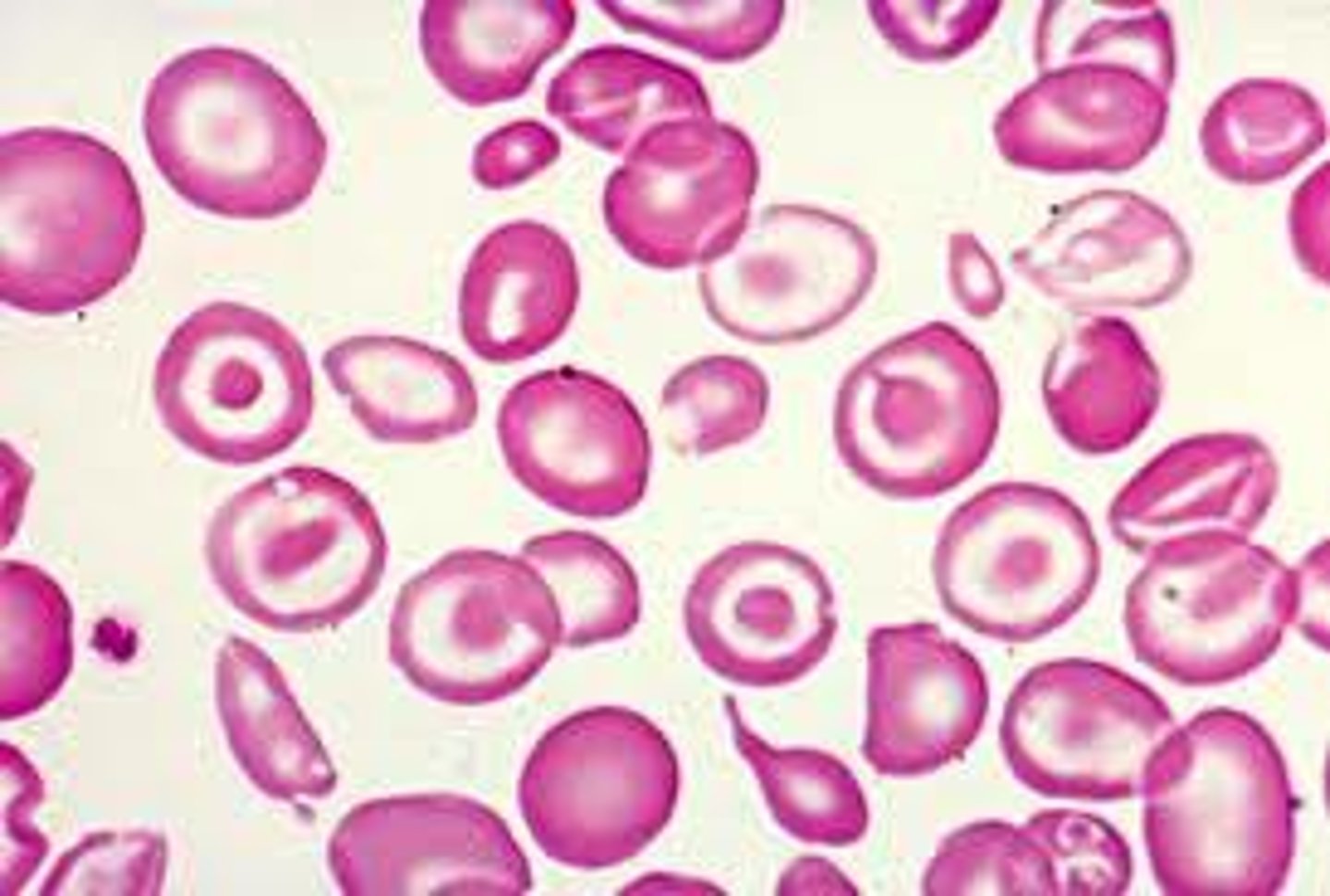
Macrocytic
If increased reticulocytes then recovery in progress: recovering from hemorrhage or hemolysis
If decreased or normal reticulocytes and megaloblastic (hyper-segmented neutrophils) then B12/ folate deficiency
If decreased or normal reticulocytes and not megaloblastic then more than just temporary DNA synthesis impairment and could be liver disease or myelodysplastic anemia
Defects in globin
quality= Thalassemia
Structure= hemoglobinopathy
Primary iron containing cells
enterocytes (intestinal absorption cells)
Ferric iron
Fe3+
- from plants
Ferrous iron
Fe2+
- from red meat
Iron absorption pathway
1. Fe3+ turned to Fe2+ by Dcyt B
2. Fe2+ enters enterocyte through DMT1
3. Some Fe2+ stored in ferritin
4. Fe2+ leaves cell and enters blood through ferroportin
5. Hephaestin turns Fe 2+ back into Fe3+ to board transferrin
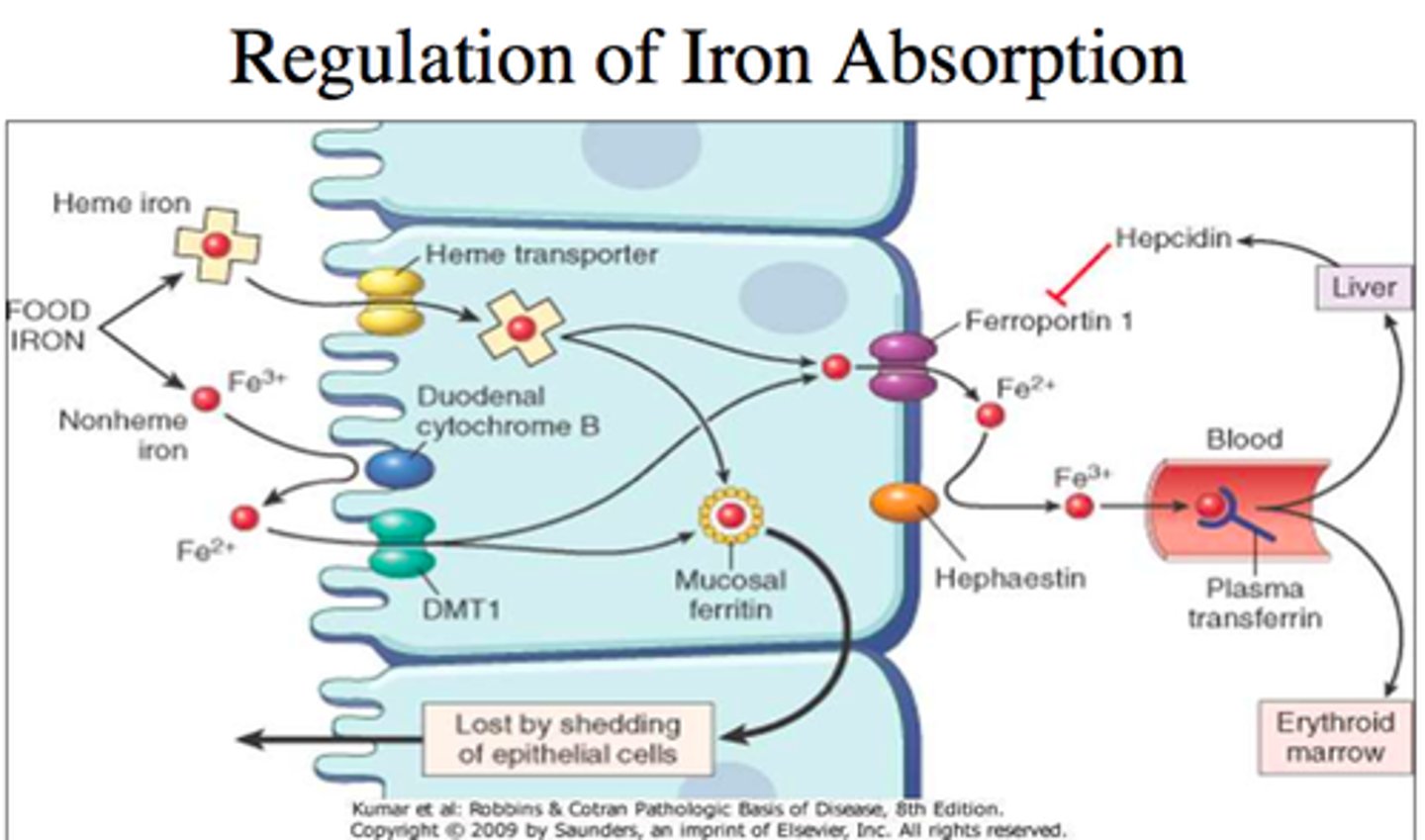
Transferrin (TIBC) testing
Amount of transferrin in blood
- if Fe is low in storage and serum then TIBC will go up as the body tries to compensate
Ferritin testing
proportional to the amount of storage Fe
- if ferritin decreases then thats the 1st indicator of Iron deficiency anemia
Serum transferrin receptor (sTFR) testing
inversely proportional to the amount of Fe in the body
- increases when body lacks Fe
Zinc Protoporphyrin
-Zn substituted for iron within Hgb when inadequate iron available; rise during iron deficiency
Hepcidin
regulatory hormone made by liver to down regulate Fe absorption
% saturation of transferrin
serum iron/TIBC x 100
-% of bus thats full
Unsaturated Iron Binding Capacity (UIBC)
TIBC - Serum iron
- number of empty seats
iron deficiency anemia iron panel
Low Iron, ferritin, and % transferrin saturation
High TIBC and UIBC
Iron depleted so stores and % of bound transferrin also decreased; UIBC and TIBC increased since binding affinity increased as compensation and # of empty seats increased without passengers
Hemochromatosis (excess iron)
High Iron, ferritin, and % transferrin saturation
Low TIBC and UIBC
Overload of Fe causes stores and saturation to be high, body down regulates TIBC to calm down and UIBC is low since not a lot of empty seats
Anemia of Chronic illness iron panel
Low iron, TIBC, and % transferrin saturation
Normal/Low UIBC
Normal/High ferritin
Fe is hidden from threat so TIBC is also down regulated to hide it, % saturation decreased since little Fe in circulation; TIBC and UIBC low since don't want people riding the bus and not many people are out to anywho
Sideroblastic anemia iron panel
High ferritin and % Transferrin saturation
Normal/ High Iron
Normal/Low TIBC and UIBC
Fe is not properly utilized so the stores and % are increased due to inability to use, TIBBC and UIBC either low or normal since body already has a lot of iron
Ferritin
short term Fe storage in liver
- gransules of sideroblast
Hemosiderin
long-term Fe storage in lover
How Fe goes into the cell:
1. transferrin brings Fe to the TfR1
2. membrane breaks off and transferrin goes inside cell through newly formed endosome
3. Acidic vessicle fuses with endosome causing the Fe to release because of acidity
4. iron enters cytoplasm through DMT1 or is stored in ferritin
5. Complex goes to cell surface and proteins are recycled and used again
DMT1
transports Fe from endosome to cytoplasm
DCyt- B
reduces Fe 3+ to Fe 2+
Ferroportin/ Ceruplasmin
In gut: Ferropoportins exports cellular Fe from enterocytes, macrophages, and hepatocytes
Outside gut: Ceruloplasmin exports cellular Fe from macrophages
- requires copper
HFE
regulates hepcidin expression
Hemojuvelin
Regulates hepcidin production
HIF- 2
Regulates EPO, DMT, DCytB, transferrin, and TfR1
Iron depletion stages
1. "Iron depletion stage"- decreased Ferritin
2. "Iron dependent erythropoiesis stage" - decreased ferritin and Serum Fe, increased TIBC
3. "Iron deficiency anemia" - decreased ferritin, Hgb, and Serum Fe, increased TIBC
Functional classifications of anemia
1.) Proliferation defects
2.) Maturation defects
3.) Survival defects
Anemia of chronic disease
Microcytic anemia with ↓ serum iron, ↓ total iron-binding capacity (TIBC), and normal or ↑ ferritin, ↓ EPO, ↑ M:E ratio
Sideroblastic anemia
Defect in 1st enzyme of heme synthesis: ALA synthesis, causing iron ring to form, can be inherited but typically acquired through lead poisoning, alcoholism, and leukemia
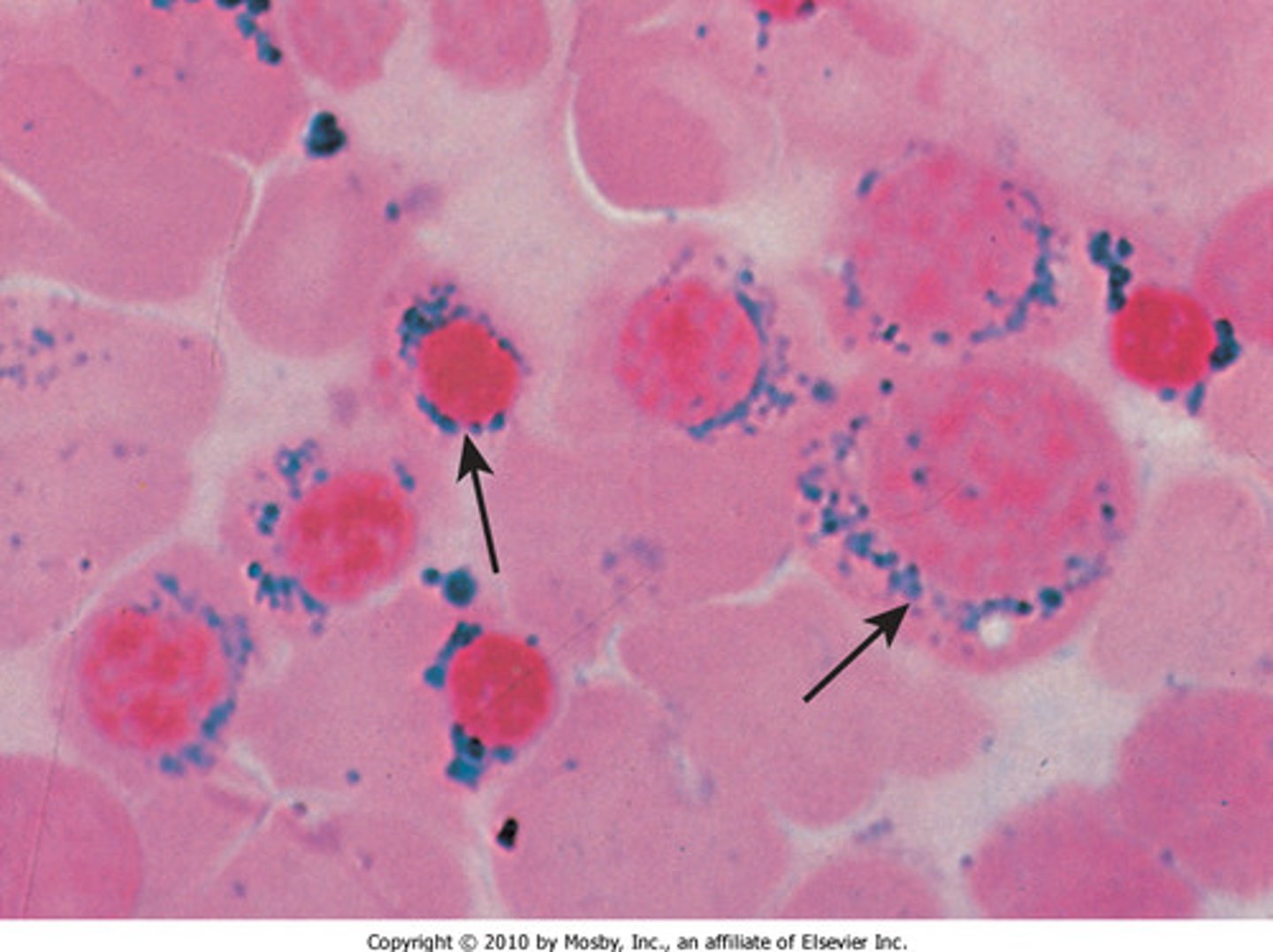
Sideroblastic anemia morphology
Microcytic/ hypochromic, ↑ total iron, ↑ ferritin, ↑ %transferrin saturation, normal UIBC and TIBC
lead poisoning sideroblastic anemia
lead accumulation in mitochondria causes issues with delta ALA dehydrogenase, causing heme synthesis impairment
Alcoholic sideroblastic anemia
Inhibits: pyridoxal phosphate, urophyrinogen decarboxylase, and ferrocheletase
Enhances: Delta ALAS
- can be macrocytic (from B12/folate deficency) or microcytic (from Fe deficency)
Sideroblastic anemia treatment
pyridoxine (B6, cofactor for ALA synthase) and Folic acid
What is hemochromatosis? how is it treated?
Iron overload (about 10x normal) that can cause tissue damage.
- low TIBC and UIBC
Treated with phlebotomy and chelation (causes Fe to go down through urine)
What are common symptoms of hemochromatosis?
Fatigue, joint pain, impotence, cardiac disease.
What genetic mutations are associated with hemochromatosis?
Transferritin receptor 2 deficiency and ferroportin deficiency
- mutations in hemojuvelin, hepcidin, and DMT1
What is a potential cause of acquired hemochromatosis?
ineffective erythropoiesis anemia, chronic transfusions, liver disease
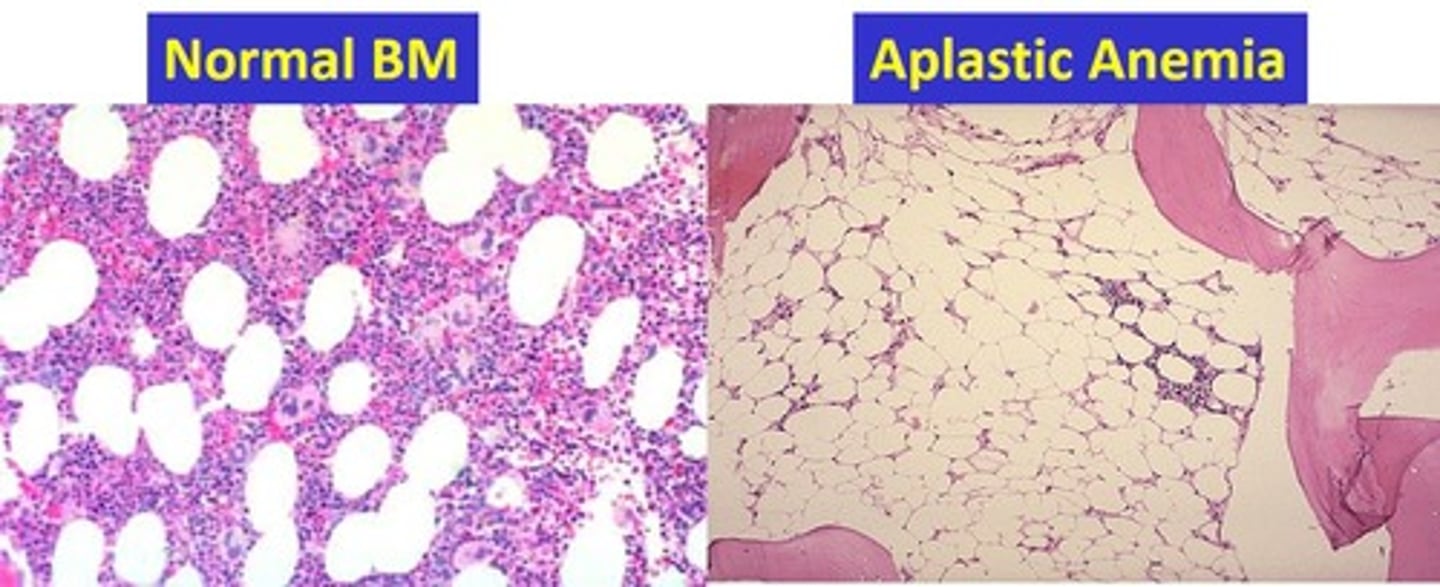
Aplastic anemia
Pancytopenia, hypocellular BM - mature cells look and function normally just low numbers, normo or macrocytic, relative lymphocytosis and neutropenia
-acquired or inherited
- acquired can be idiopathic
Fanconi Anemia
Inherited form of aplastic anemia, autosomal recessive trait causes chromosomal defect
- impaired tissue development, retardation, weird thumbs, short stature
Other forms of inherited AA
Dyskeratosis Congenita and Congenital Amegakaryocytic thrombocytopenia
Myelophthisic anemia
Marrow infiltration by fibrotic, neoplastic, or granulomatous cells (can be cancer)
- takes up BM space, causing pancytopenia, increase immature cells in blood, and bizarre platelets
Congenital Dyserythropoietic Anemia (CDA)
abnormal and ineffective erythropoiesis characterized by bi-nucleated RBC precursors
bone marrow is normocellular or hypercellular, but peripheral blood is pancytopenic with basophilic stippling, poilk, and anisocytosis
Diamon Blackfan Anemia
Rare inheritted RBC aplasia due to defect in erythroid progenitor
-in very young children, causes long thumbs
- ↑ EPO levels, BM normocellular with erythroid hypoplasia
-Macrocytic, normochromic, reticulocytopenia
-Treated with RBC transfussions and steroids
Pure Red Cell Aplasia
Targets erythropoesis; normocytic, normochromic anemia
-corrected retic= <1%
-inheritted or acquired through Igs to EPO or T-cell mediated inhibitted erythropoesis
Transient Erythroblastopenia of Childhood (TEC)
transient severe anemia in young children triggered by viral infection
- Ig or T cell mediated repression of RBC precursors
-Not related to anemia
Anemia of Chronic Kidney disease (ACKD)
Decreased erythropoetin, decreased RBC lifespan, Iron and folate deficiency
-Macro or microcytic, schistocytes
Endocrine Abnormalities Causing Hypoproliferative disorders
Decreased erythropoesis and decreased EPO
- normocytic, normochromic
-Erythroid hypoproliferation due to unstable hormone levels