Bonding and properties of triglycerides
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
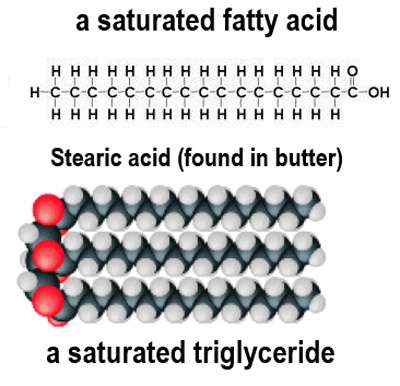
What are saturated fatty acids characterized by?
They have only single bonds between carbon atoms and contain the maximum number of hydrogen atoms.
At room temperature, lipids containing saturated fatty acids generally form what?
Fats.
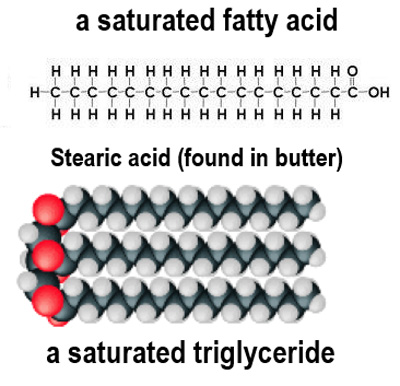
Why do saturated fatty acids have a higher melting point?
Their straight tails can pack closely together, leading to stronger forces of attraction.
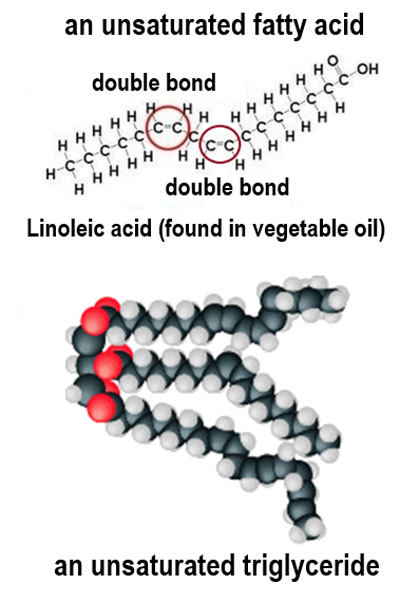
What distinguishes unsaturated fatty acids from saturated ones?
Unsaturated fatty acids have one or more double bonds between carbon atoms.
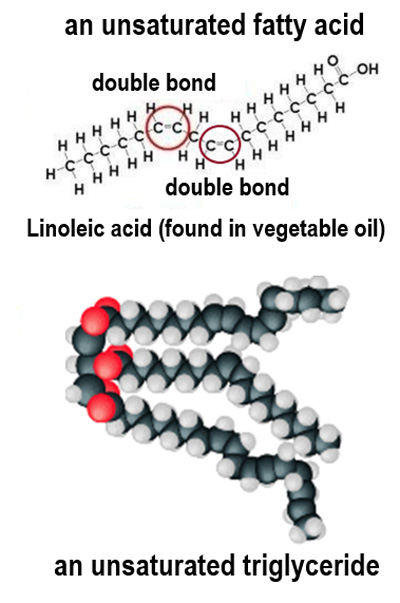
What is the state of lipids containing unsaturated fatty acids at room temperature?
They are usually oils.
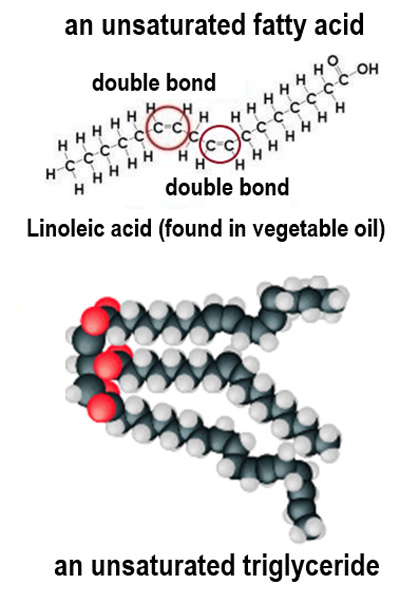
Why do unsaturated fatty acids have a lower melting point?
They have kinks in their tails, preventing close packing and resulting in weaker forces of attraction.
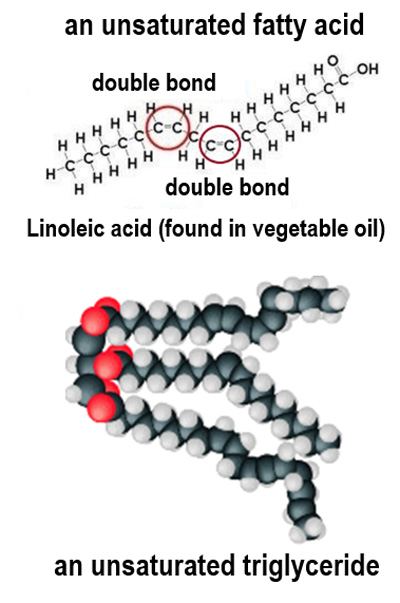
How many fewer hydrogen atoms does a fatty acid contain for each carbon-carbon double bond?
Two fewer hydrogen atoms.