enantiomers, diastereomers, identical compounds, or constitutional isomers
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
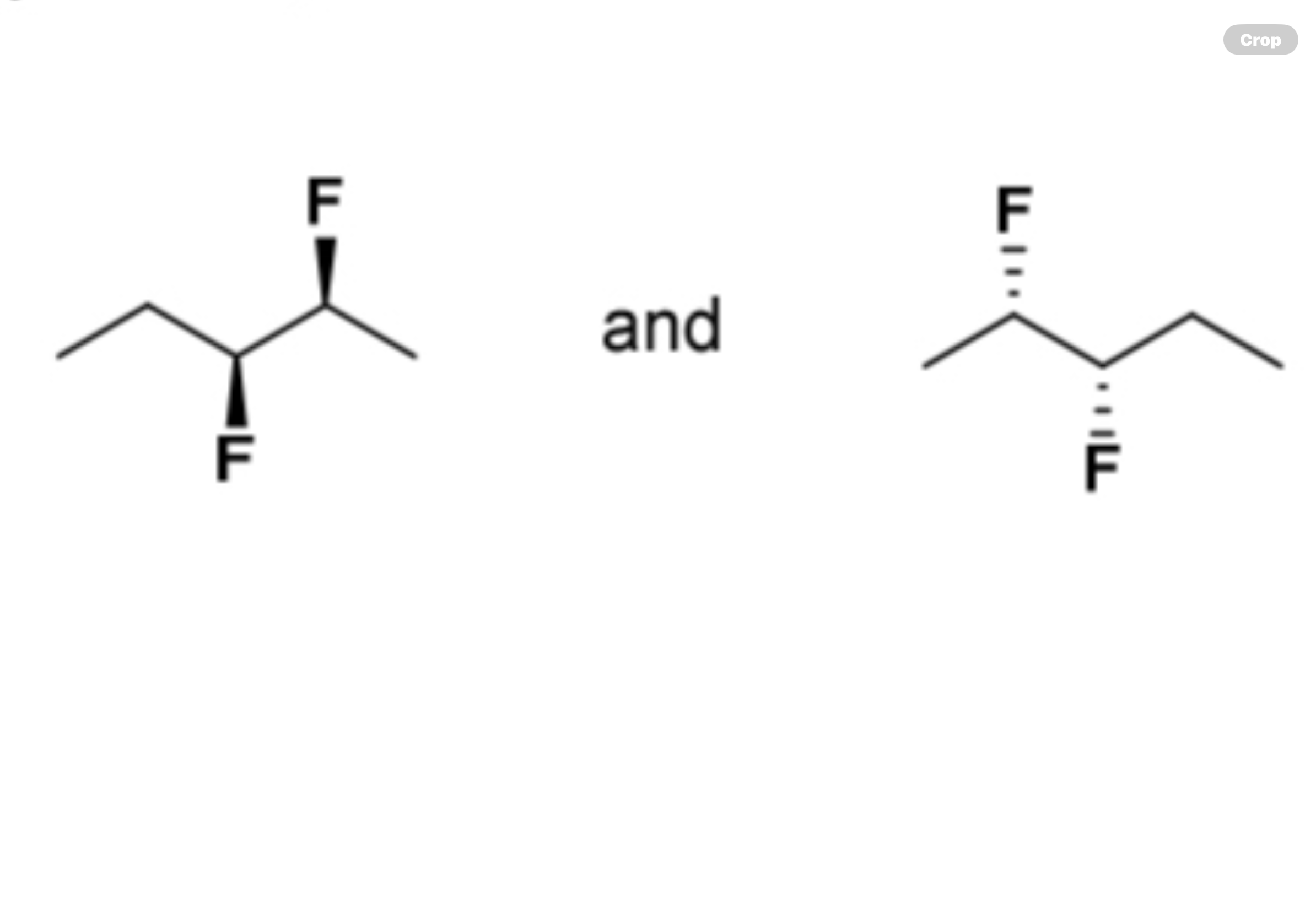
enantiomers, diastereomers, identical compounds, or constitutional isomers?
identical
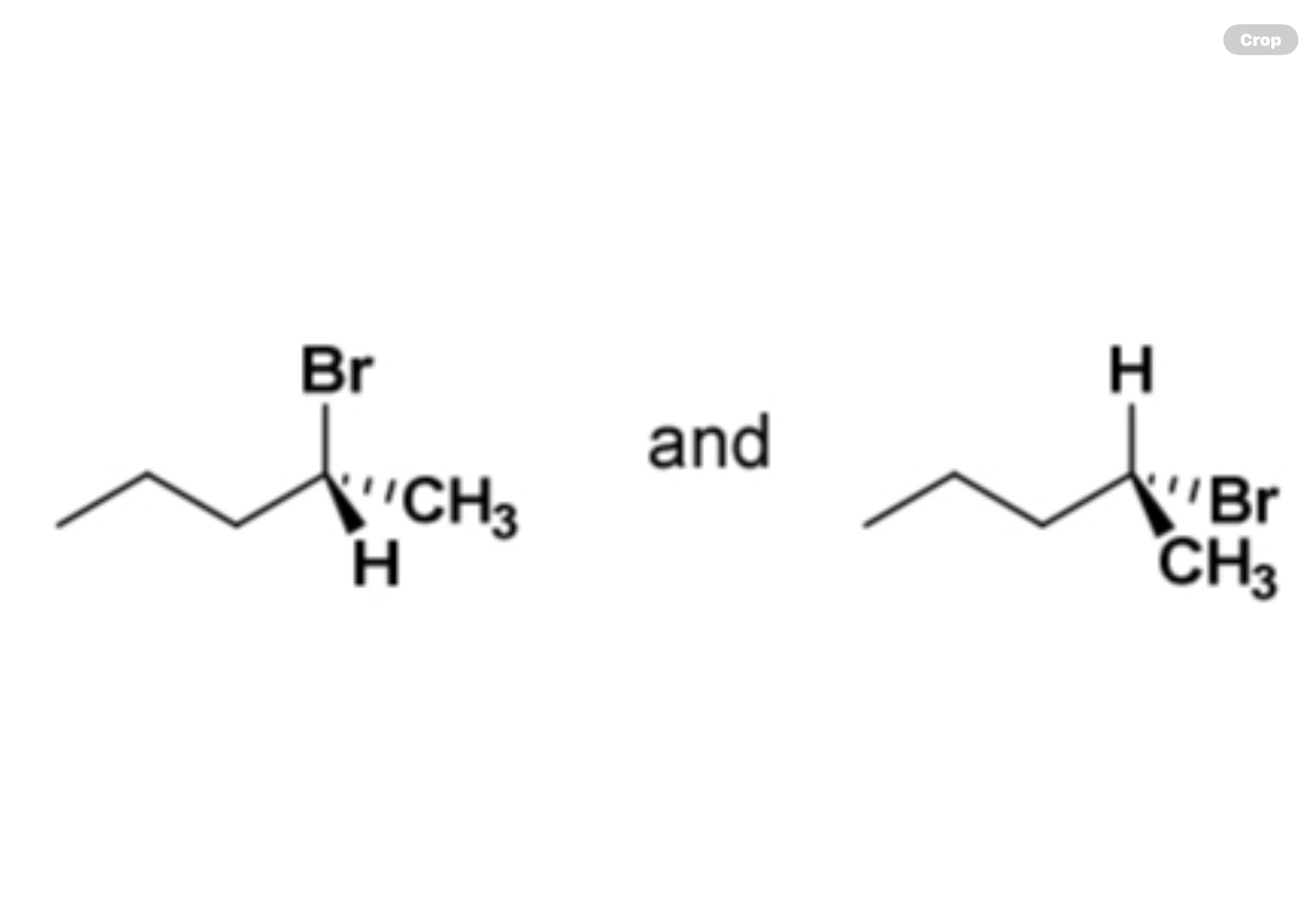
enantiomers, diastereomers, identical compounds, or constitutional isomers?
identical
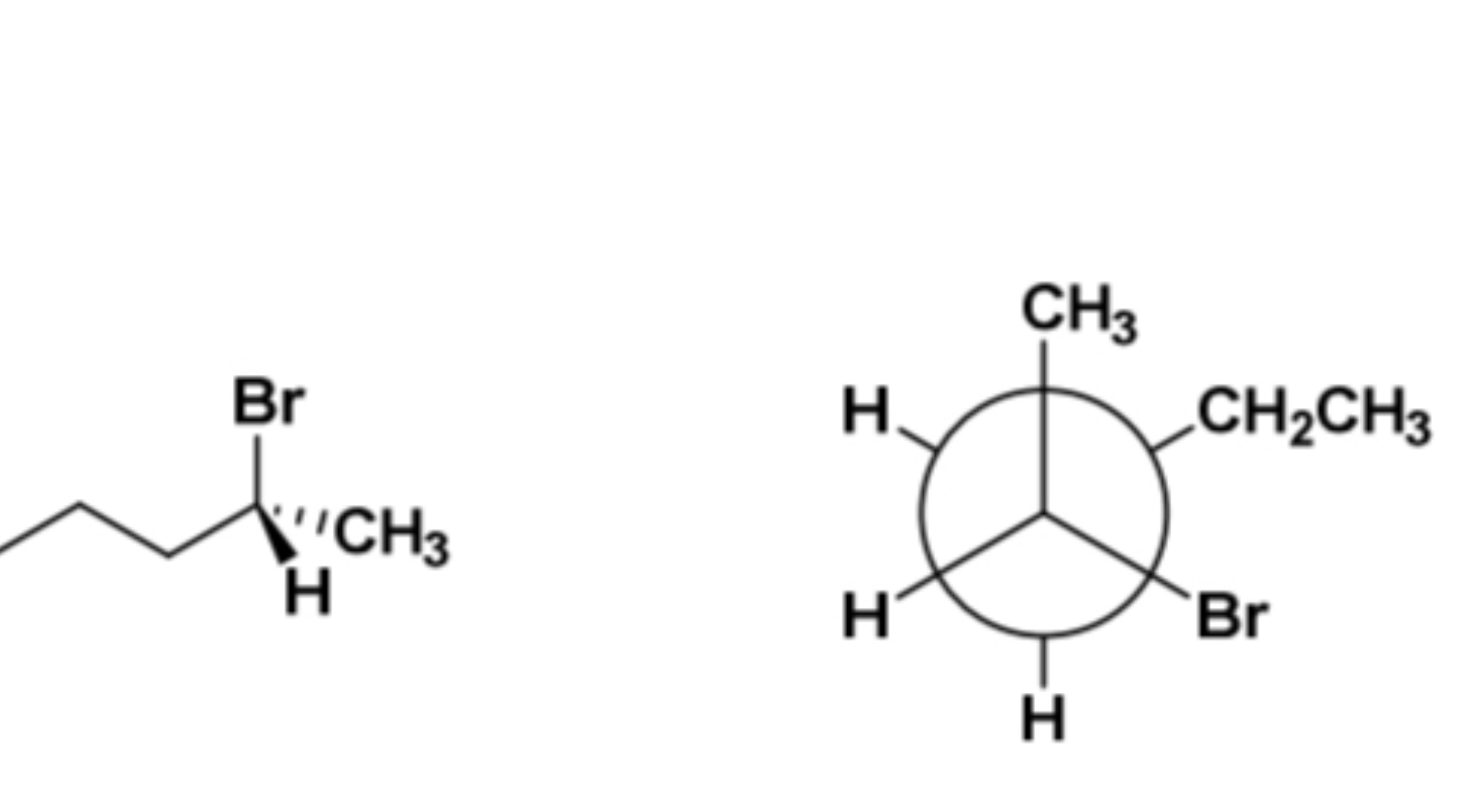
enantiomers, diastereomers, identical compounds, or constitutional isomers?
enantiomers
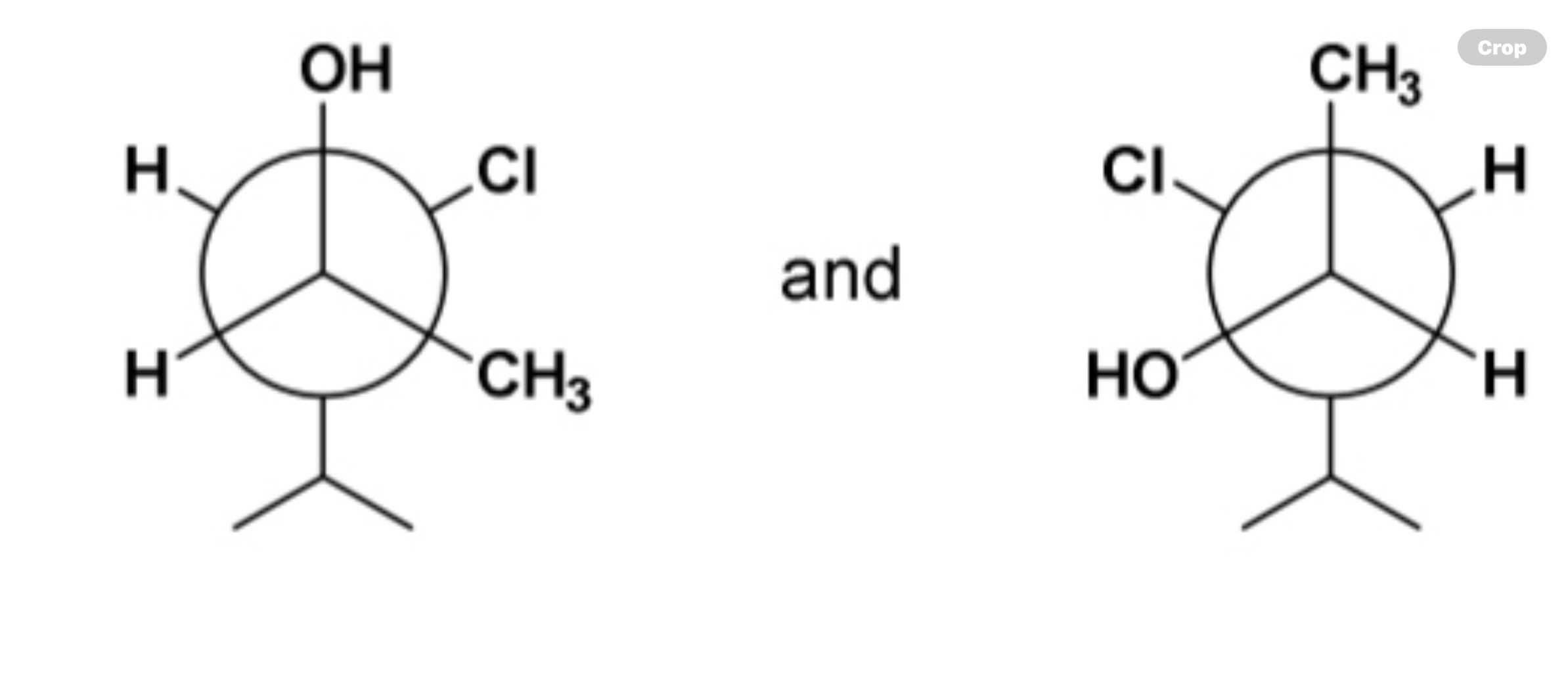
enantiomers, diastereomers, identical compounds, or constitutional isomers?
diastereomers
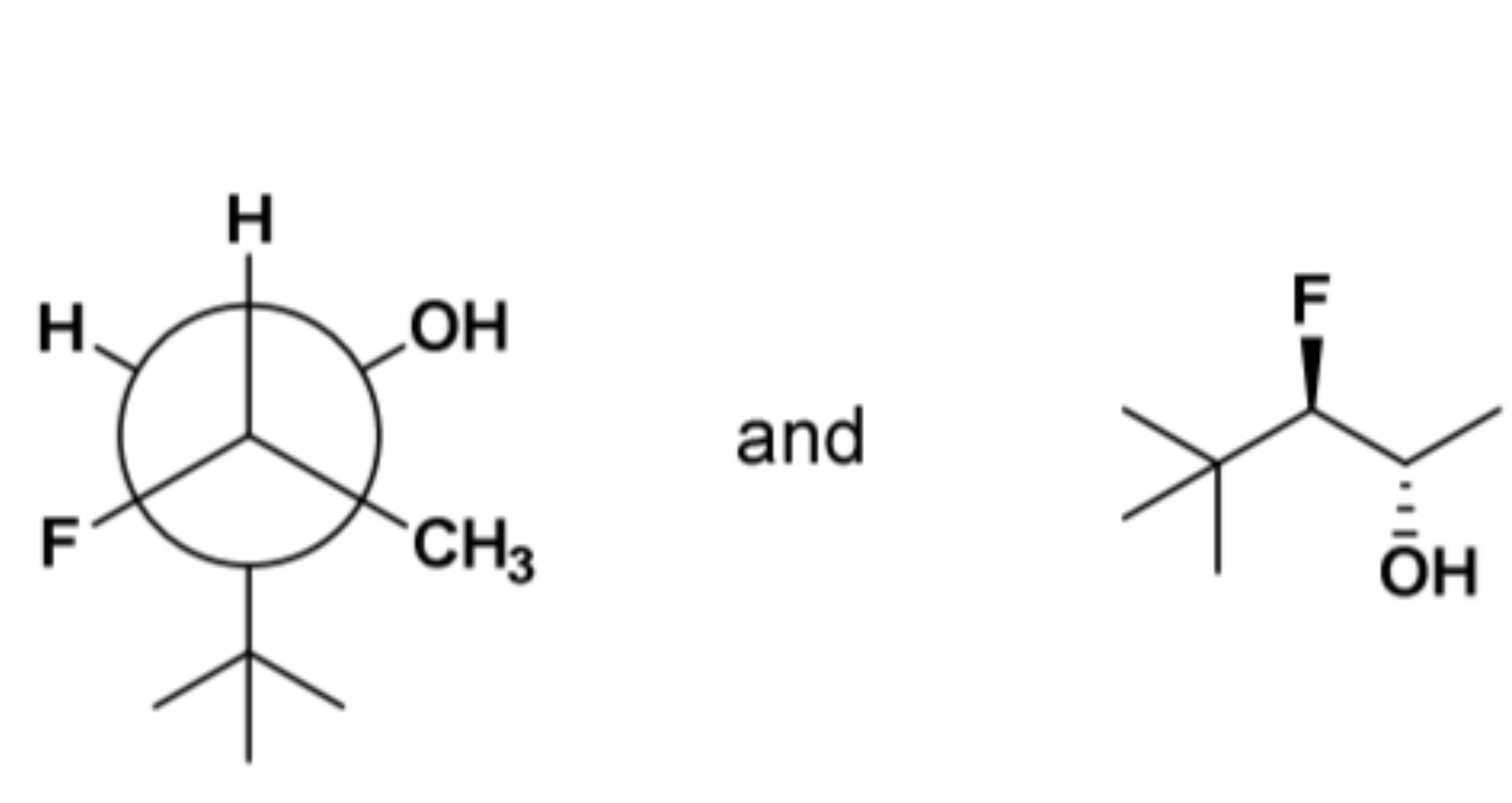
enantiomers, diastereomers, identical compounds, or constitutional isomers?
constitutional isomers
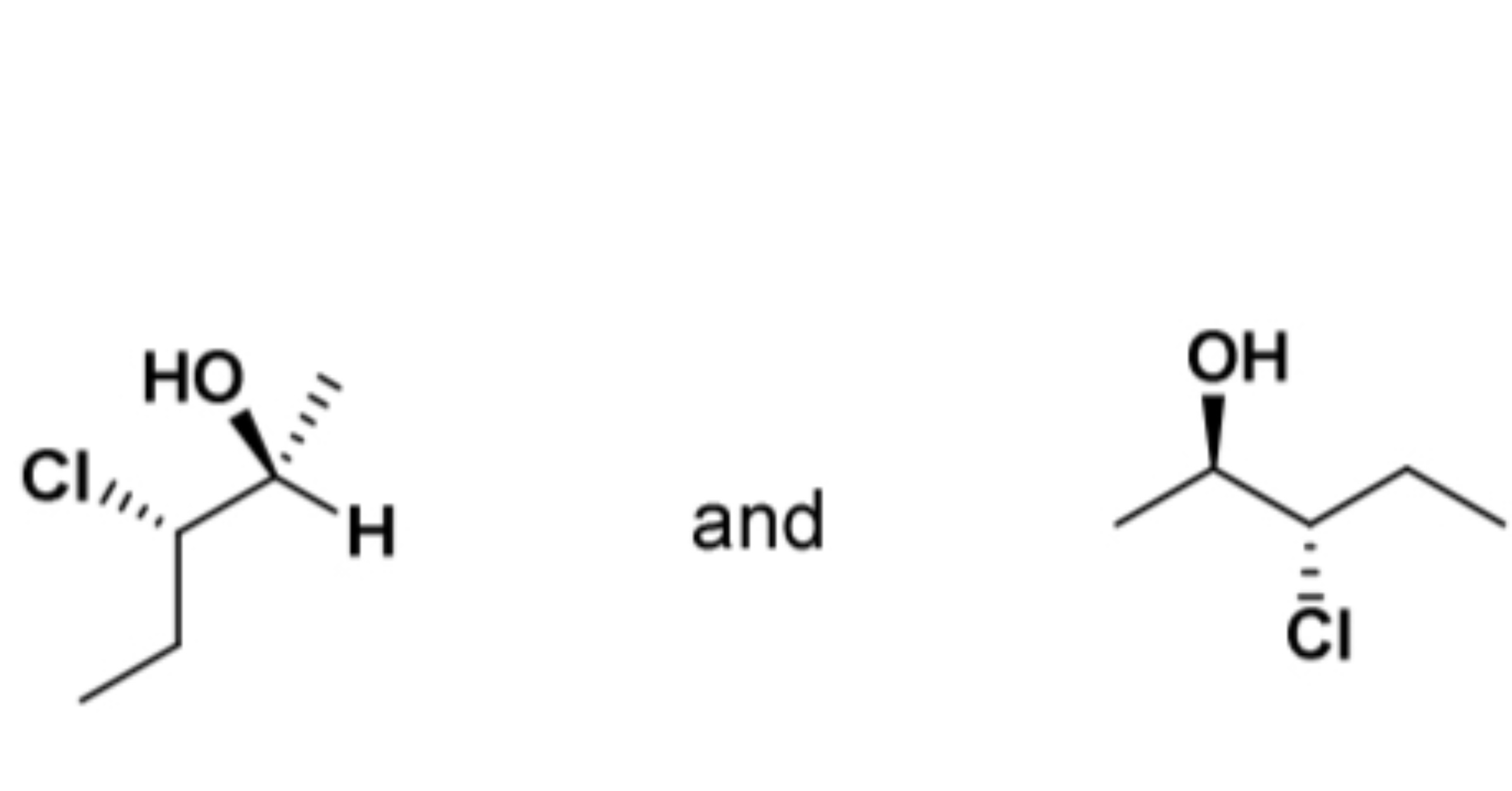
enantiomers, diastereomers, identical compounds, or constitutional isomers?
identical
enantiomers
Definition: Non-superimposable mirror images of each other.
Key feature:
All chiral centers are opposite (R ↔ S).
They have identical physical properties (melting point, boiling point, density, etc.) except for how they interact with plane-polarized light and with chiral environments.
Example:
(R)-2-butanol and (S)-2-butanol.
🪞 Think of them like your left and right hands — mirror images that can’t perfectly overlap.
diastereomers
Definition: Stereoisomers that are not mirror images of each other.
Key feature:
They differ at one or more (but not all) chiral centers.
They have different physical and chemical properties.
Example:
(2R,3R)-2,3-dibromobutane vs (2R,3S)-2,3-dibromobutane.
🙃 Not mirror images, but still differ in 3D arrangement.
identical compounds
Definition: Representations of the same molecule — same connectivity and same 3D arrangement.
Key feature:
All atoms are connected in the same way, and all stereocenters have the same configuration.
Example:
Two drawings of (R)-lactic acid, just rotated or flipped differently in space.
🧩 Think: Different drawings, same molecule.
constitutional isomers
Definition: Compounds with the same molecular formula but different connectivity of atoms.
Key feature:
Atoms are bonded in different orders — different skeletons or functional groups.
Often have very different physical and chemical properties.
Example:
C₄H₁₀ → butane and isobutane (2-methylpropane).
🔗 Different connections, same number of atoms.
nonsuperimposable
no matter how you rotate or flip one object, it cannot perfectly overlap with the other — all parts don’t line up exactly in 3D space
Think of your left and right hands:
They’re mirror images of each other.
But if you try to place one hand on top of the other, your thumbs and pinkies are on opposite sides — they don’t match up exactly.
That means your hands are non-superimposable mirror images — just like enantiomers in chemistry.