Chapter 10: Free-Radical Reactions, Main-Group Organometallic Compounds, And Carbenes
1/57
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
58 Terms
What are peroxides?
compounds of the general structure R—O—O—R
What is the peroxide effect?
Reversal of regioselectivity in HBr addition
What promotes the peroxide effect?
Light
Are promoted reactions fast?
Yes, faster than HBr addition in the absence of peroxides
Do peroxides affect HI and HCI?
The regioselectivity of HI or HCl addition to alkenes is not affected by the presence of peroxides
What are the rules for fishhook notation?
Rules for unpaired electrons:
A fishhook tail must be located on every unpaired electron of the reactant that is involved in a transformation.
A fishhook barb must be located on every atom of the reactant that is to gain an unpaired electron in the product.
Rules for electron-pair bonds:
The tails of two fishhooks must be located on every bond of the reactant that is to break homolytically.
Two fishhook barbs must be shown together in the reactant at the location of a new electron-pair bond in the product.
What do free-radical chain reactions involve?
Involves free-radical intermediates and consists of the following three fundamental reaction steps:
Initiation steps
Propagation steps
Termination steps
What are the initiation steps?
The free radicals that take part in subsequent steps of the reaction are formed from a free-radical initiator , which is a molecule that undergoes homolysis with particular ease
Peroxides, such as di-tert-butyl peroxide, are frequently used as free-radical initiators
The first initiation step in the free-radical addition of HBr to an alkene is the homolysis of the peroxide
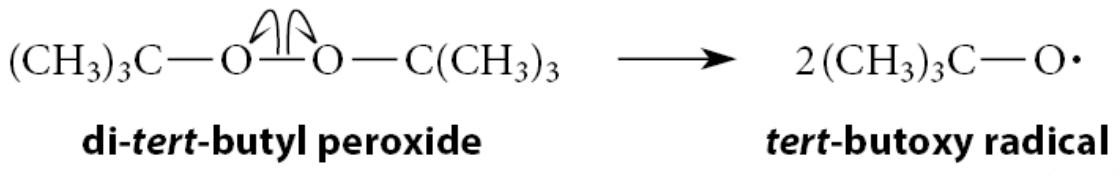
Sometimes heat or light initiates a free-radical reaction. This usually happens because the additional energy causes homolysis of the free-radical initiator—or, in some cases, the reactants themselves—into free radicals
A second initiation step occurs in the free-radical addition of HBr to alkenes: the removal of a hydrogen atom from HBr by the tert-butoxy free radical that was formed in the first initiation step
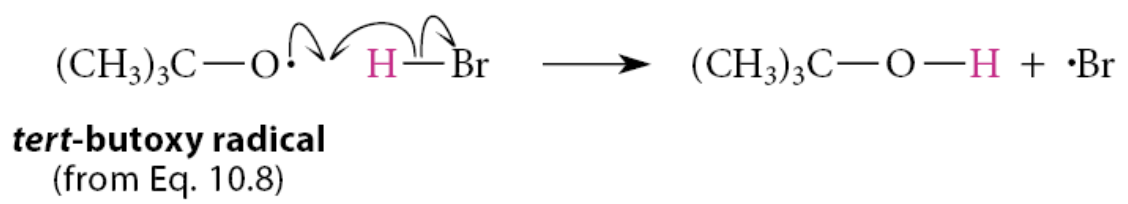
What are the propagation steps?
Radicals react with nonradical starting materials to give other radicals; starting materials are consumed, and products are formed
The first propagation step of free-radical addition of HBr to an alkene is the reaction of the bromine atom generated with the π bond
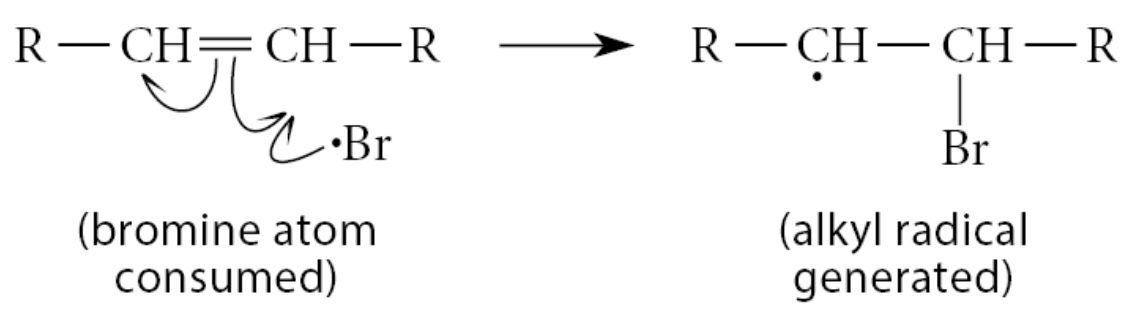
The second propagation step is another atom abstraction reaction—namely, removal of a hydrogen atom from HBr by the free-radical product to give the addition product and a new bromine atom
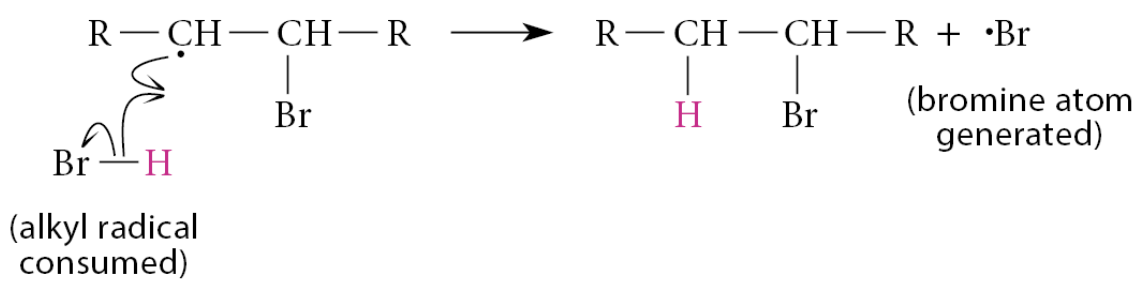
These two propagation steps continue in a chainlike fashion until the reactants are consumed. That is, the product free radical of one propagation step becomes the starting free radical for the next propagation step
What are the termination step?
Two radicals react to give nonradical products
In these reactions, the chain-propagating radicals recombine to form by-products
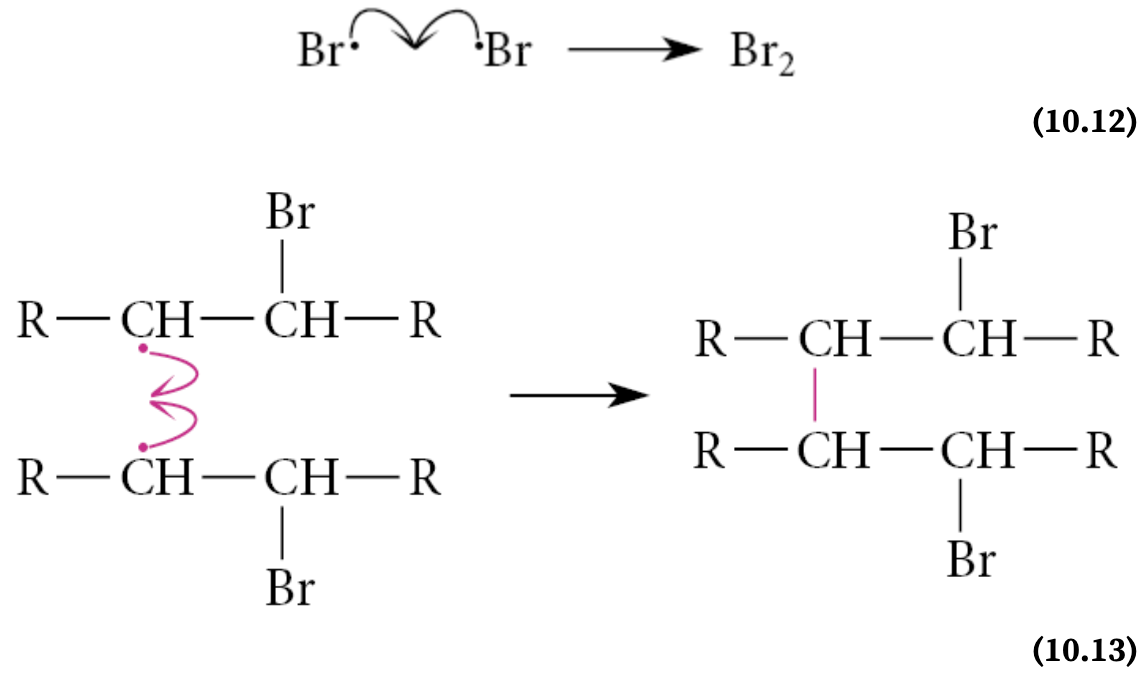
The recombination reactions of free radicals are in general highly exothermic—that is, they have very favorable, or negative, ΔH° values
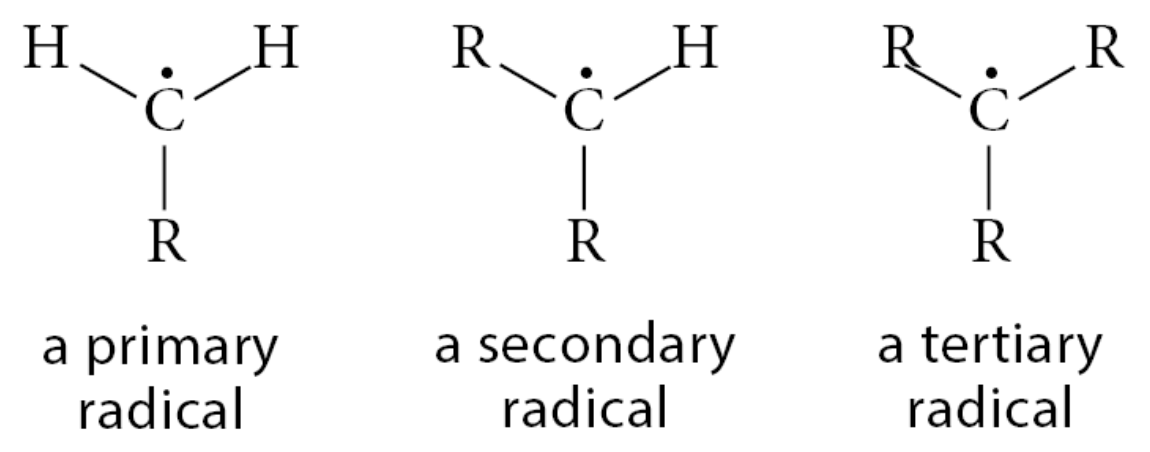
What is the stability of free radicals?
Tertiary > Secondary > Primary
10.2 Conversion of Internal Alkynes into Trans Alkenes
What does the reaction of an internal alkyne with a solution of an alkali metal in liquid ammonia give?
A trans alkene
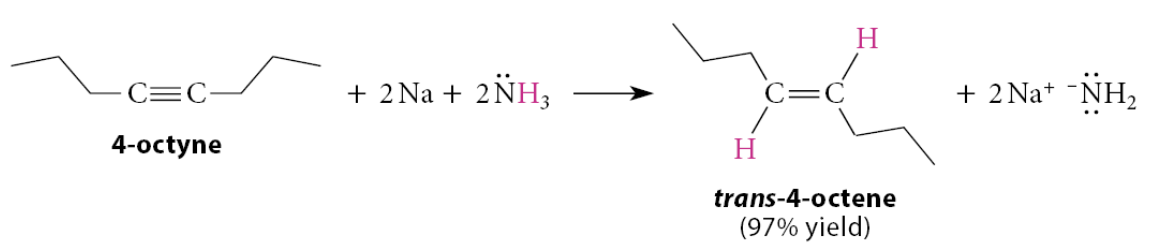
What are the steps for this reaction?
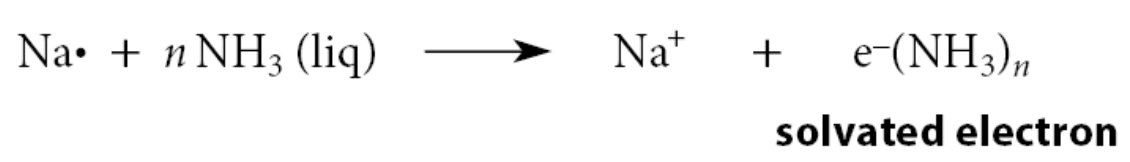
If sodium or other alkali metals are dissolved in pure liquid ammonia, a deep blue solution forms that contains electrons associated noncovalently with ammonia (solvated electrons)
The reaction of solvated electrons with an alkyne begins with the addition of an electron to the triple bond. The resulting species has both an unpaired electron and a negative charge. Such a species is called a radical anion
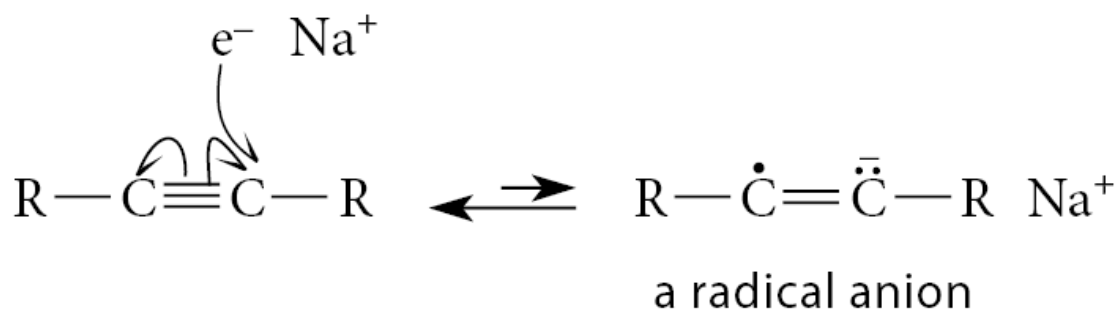
The radical anion is such a strong base that it readily removes a proton from ammonia to give a vinylic radical—a radical in which the unpaired electron is associated with one carbon of a double bond. This reaction is a Brønsted acid–base reaction and not a radical reaction
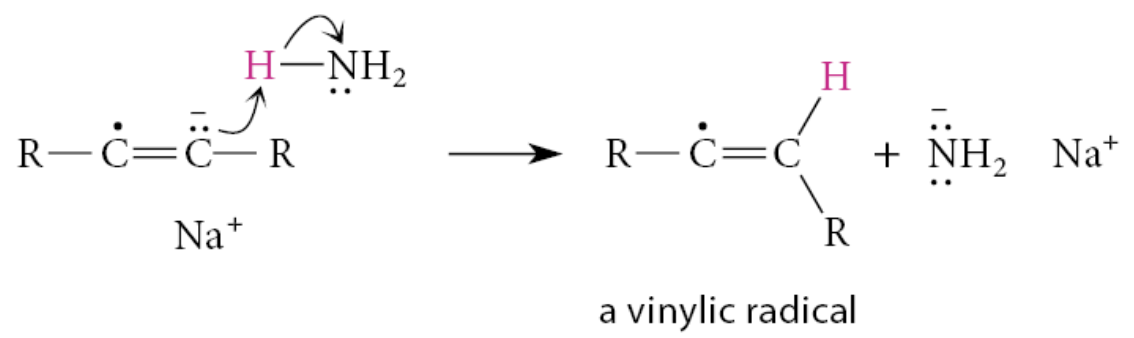
Next, the vinylic radical accepts an electron to form an anion
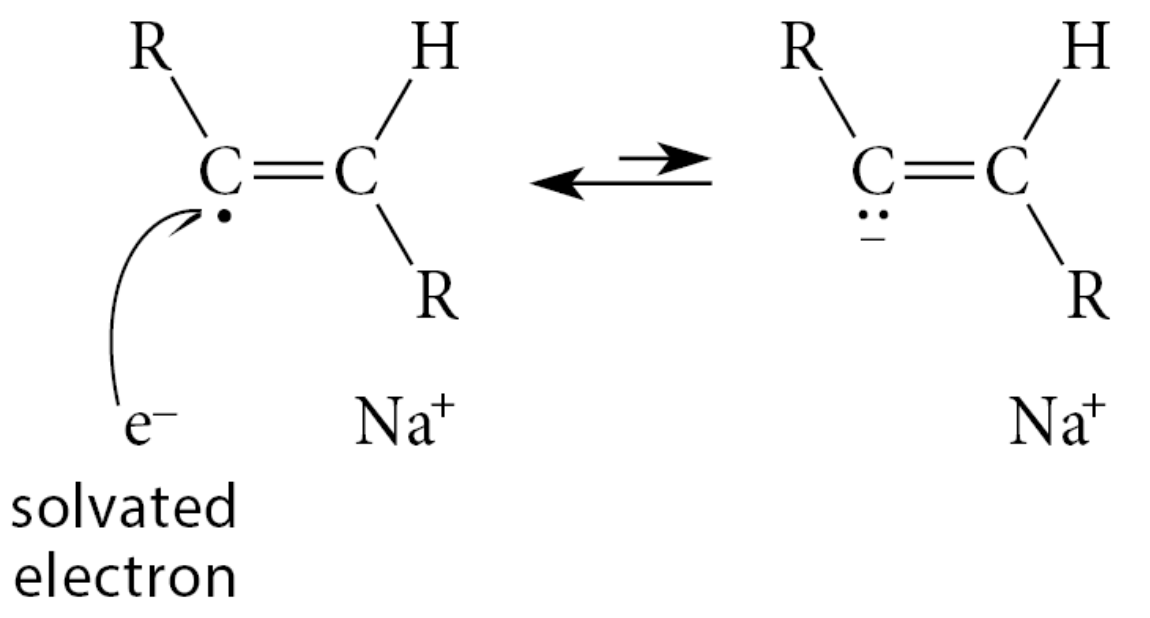
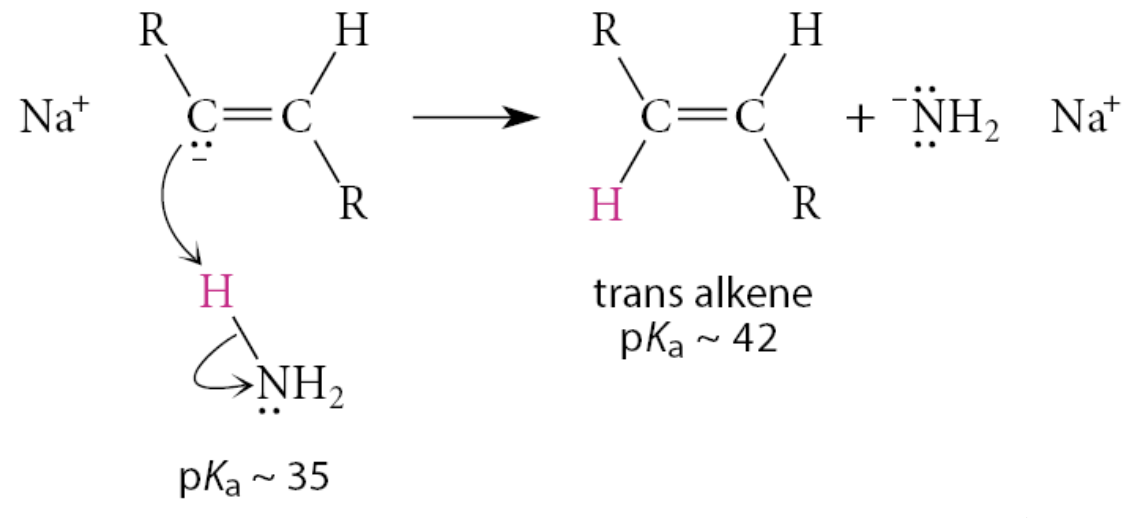
The anion formed is also more basic than the amide anion and readily removes a proton from ammonia in another Brønsted acid–base reaction to complete the addition
Does the Na/NH3 reaction with alkynes work well on 1-alkynes?
The Na/NH3 reaction with alkynes does not work well on 1-alkynes unless certain modifications are made in the reaction conditions
10.3 Polymers; Free-Radical Polymerization of Alkenes
What are polymers?
Very large molecules composed of repeating units
What are polymerization reactions?
Small molecules known as monomers react to form a polymer
What is source-based name?
The polymer is named with the prefix poly followed by the name of the monomer in parentheses
In polymers, what does the n subscript indicate?
The subscript n means that a typical poly(ethylene) molecule contains a very large number of repeating units.
What are the steps for free-radical polymerization?
Note: ethylene is the example here
The reaction is initiated when a radical R·, derived from peroxides or other initiators, adds to the double bond of ethylene to form a new radical.
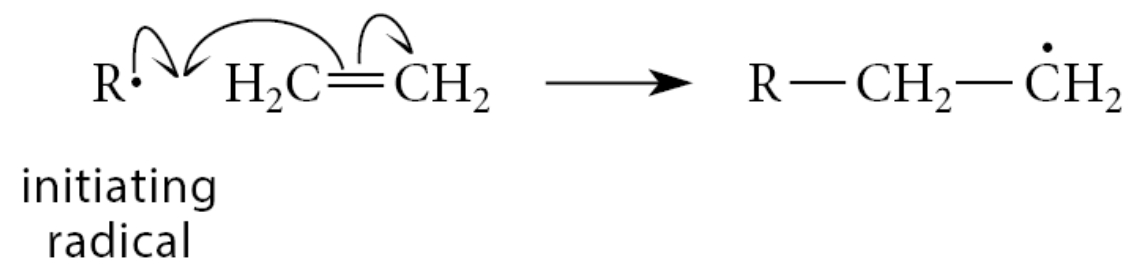
The first propagation step of the reaction involves addition of the new radical to another molecule of ethylene

This process continues indefinitely as long as the ethylene monomer is present in high concentration
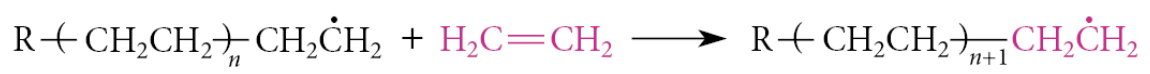
When the ethylene monomer is exhausted, termination reactions begin to occur. An example of a termination reaction is the radical recombination of two radical chains to form a large nonradical product:
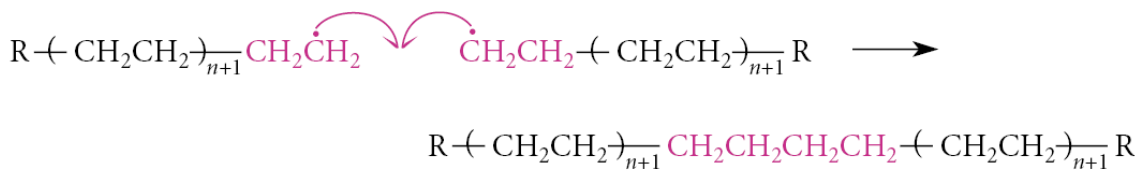
In terms of stereochemistry, what happens when the monomer has the form ═H2C═CH—X?
Three stereochemical situations (called tacticity ) are typically encountered
What are the three stereochemical situations?
In an isotactic polymer, the asymmetric carbon stereocenters have the substituents (methyl groups in this case) on the same side of the polymer chain.

In a syndiotactic polymer, the asymmetric carbon stereocenters have the substituents alternating regularly on opposite sides of the polymer chain.

Both isotactic and syndiotactic polymers are classified as stereoregular polymers.
In an atactic polymer, the configurations of the stereocenters are randomly distributed. These polymers are classified as stereorandom polymers.
In free-radical polymerization, what is the stereochemistry that is generally yielded?
Free-radical polymerization generally yields atactic polymers because the free-radical mechanism provides no control of stereochemistry
10.5 Organometallic Compounds. Grignard Reagents and Organolithium Reagents
What are organometallic compounds?
Compounds that contain carbon–metal bonds
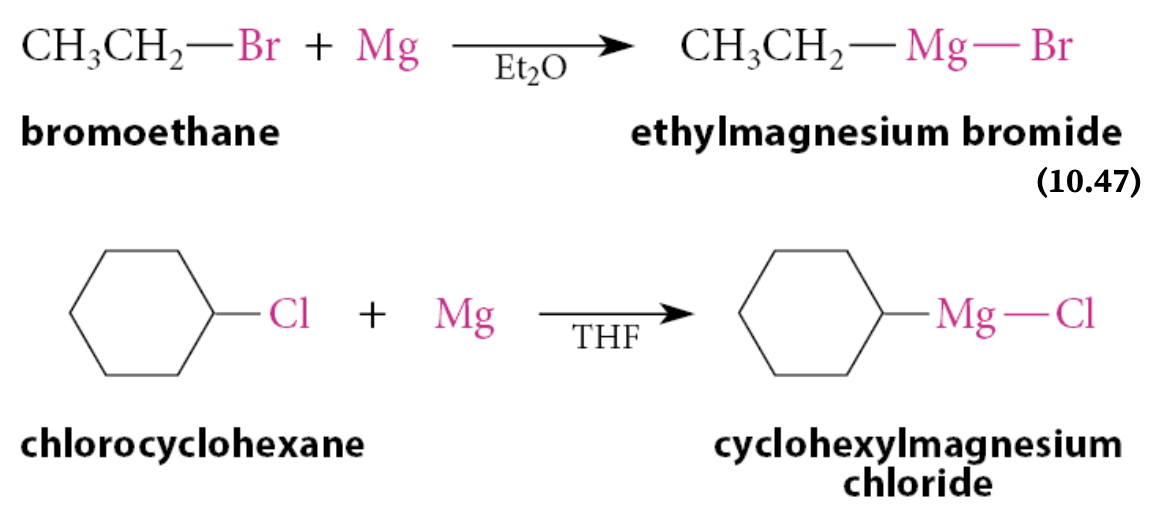
What are Grignard reagents?
A compound of the form R—Mg—X, where ═X═Br, Cl, or I
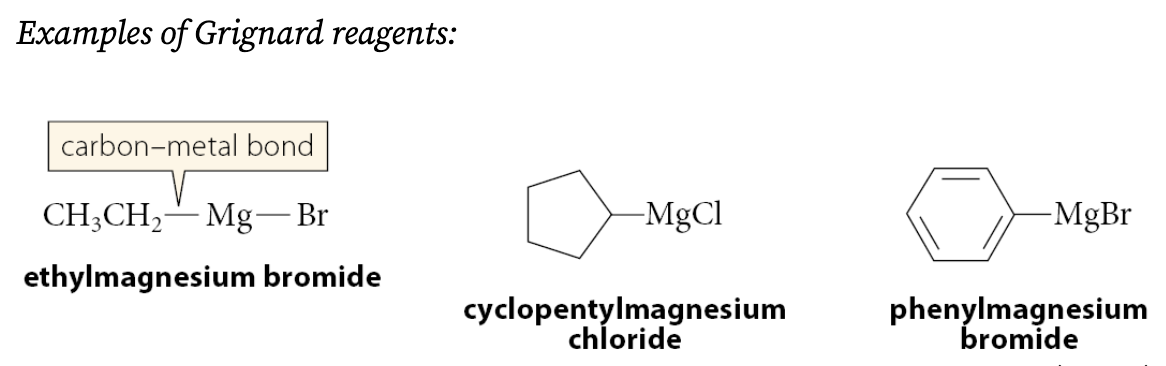
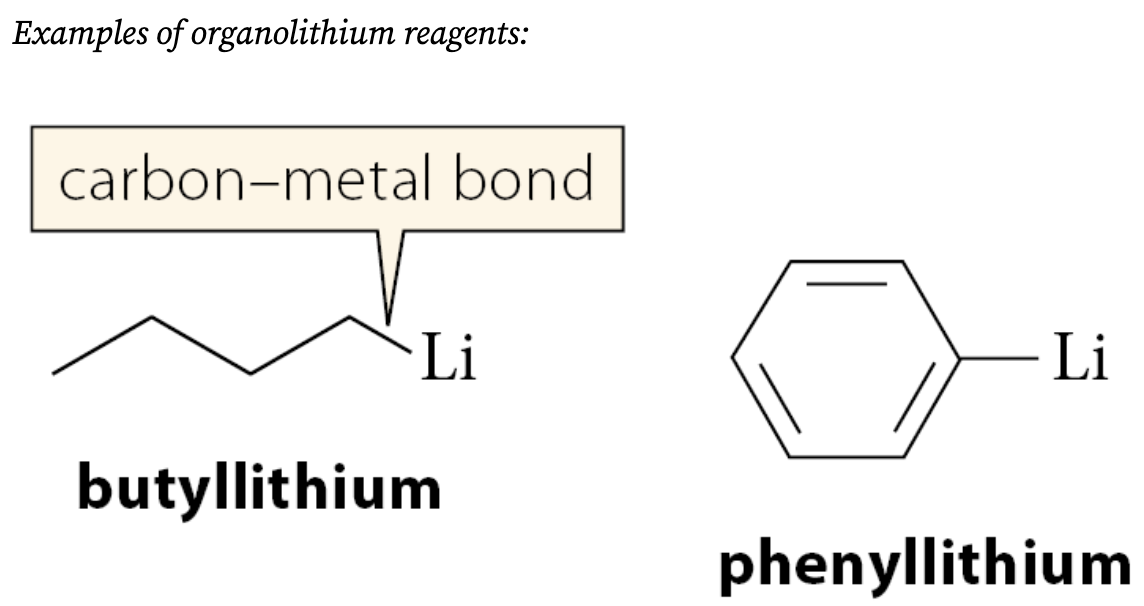
What are organolithium reagents?
Compounds of the form R—Li
What must be used for the formation of Grignard reagents?
Anhydrous ether solvents must be used for the formation of Grignard reagents
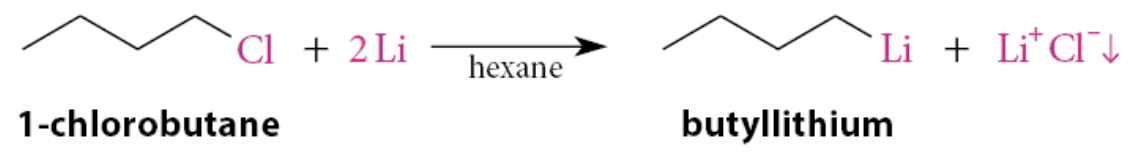
What solvents are organolithium reagents typically formed in?
Typically formed in hydrocarbon solvents such as hexane
What do Grignard and organolithium reagents react violently with?
Oxygen and vigorously with water, alcohols, and other compounds that have even weakly acidic hydrogens
What are carbanions?
Such a carbon, bearing three bonds, a nonbonding electron pair, and a negative formal charge
How do Grignard and organolithium reagents react?
Grignard and organolithium reagents react as if they were carbanions

R-MgX and R-Li are strong what?
Strong bases
What do Grignard and lithium reagents react instantaneously with?
Grignard and lithium reagents are such strong bases that they react instantaneously with even weak acids such as water or alcohols
The products of such a reaction are the hydrocarbon—that is, the conjugate acid of the organometallic “carbanion”—and the conjugate base of the proton source—hydroxide ion (if the acid is water) or an alkoxide ion (if the acid is an alcohol)
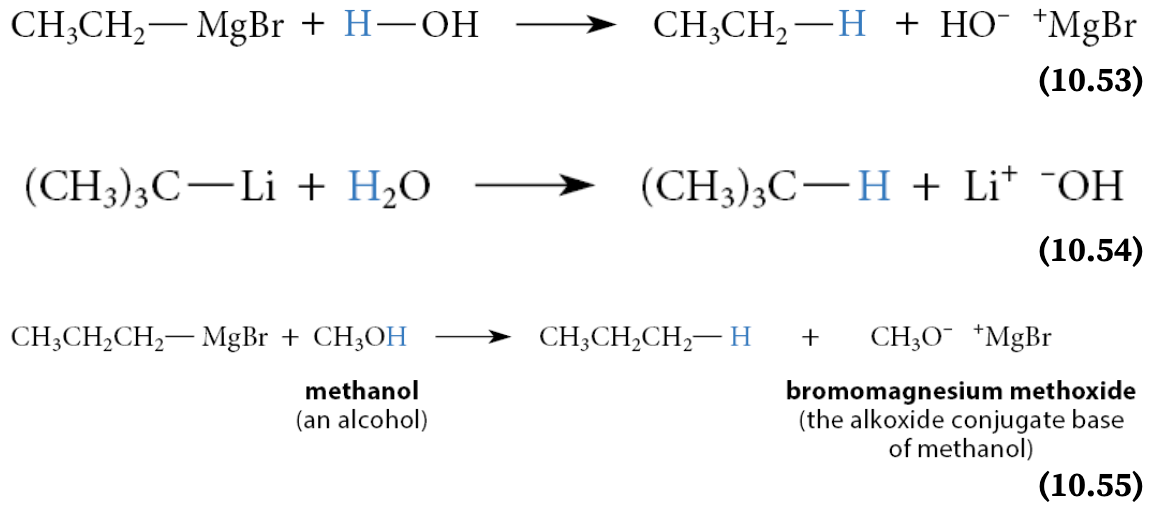
Each of these reactions can be represented with the curved-arrow notation as if it were the reaction of a carbanion base with the proton of water or alcohol:
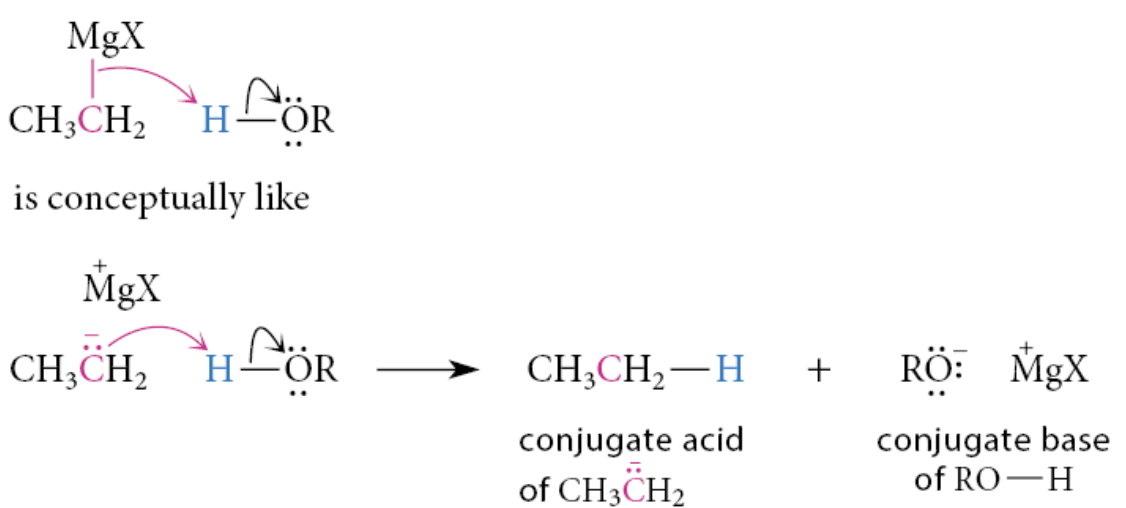
What is protonolysis?
Any reaction with the proton of an acid that breaks chemical bonds
The relative acidity of alkynes and the basic character of Grignard reagents play a role in the method usually used to prepare what?
Acetylenic Grignard reagents (reagents with the general structure R—C≡C—MgBr) and acetylenic lithium reagents (reagents with the general structure R—C≡C—Li)
Acetylenic Grignard reagents are accessible by what?
By the acid–base reaction between a 1-alkyne and another Grignard reagent
Methylmagnesium bromide or ethylmagnesium bromide are often used for this purpose because the formation of a gaseous by-product (ethane in this example) ensures the irreversibility of the reaction
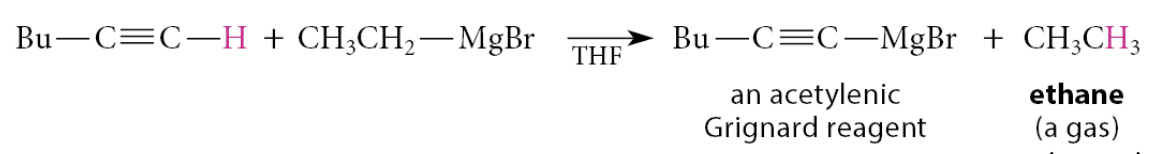
The reaction is really just another Brønsted acid–base reaction:
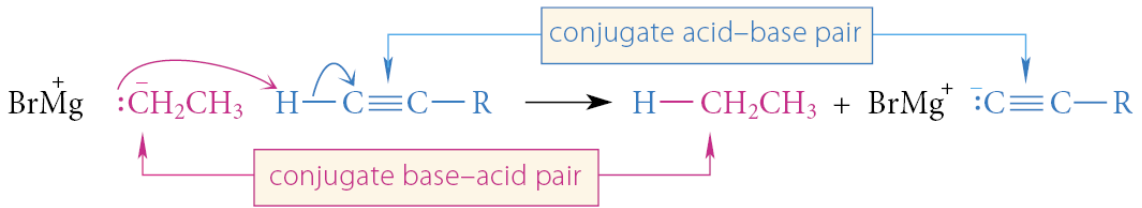
In this reaction, a stronger base (ethyl magnesium bromide, which resembles the ethyl anion) reacts with a stronger acid (a 1-alkyne) to give a weaker acid (ethane) and a weaker base (the alkynyl magnesium bromide, which resembles the acetylenic anion).
10.6 Acetylenic Anions
What can alkynes be converted into?
Alkynes are acidic enough that they can be converted into their conjugate-base anions by sufficiently strong bases
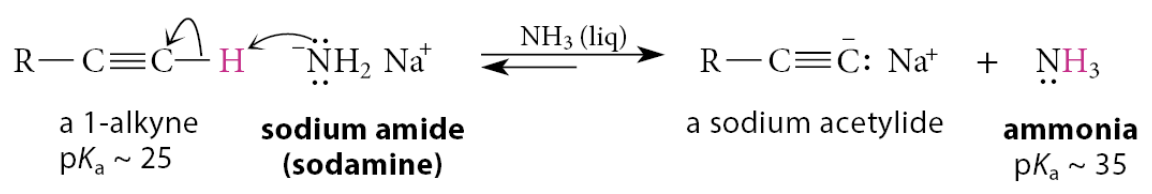
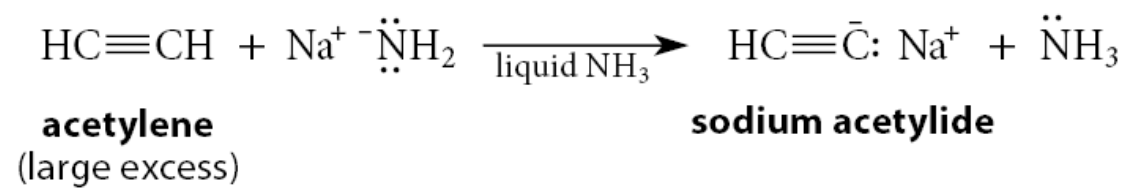
How can the monosodium salt of acetylene itself be prepared?
The monosodium salt of acetylene itself can be prepared by the same reaction using a large excess of acetylene to ensure that the di-anion doesn’t form:
Solutions of the potassium acetylide can be formed by what?
Solutions of the potassium acetylide can be formed in tetrahydrofuran (THF) solution (or in solutions of other ethers) by reaction with potassium hydride, a commercially available alkali-metal hydride:
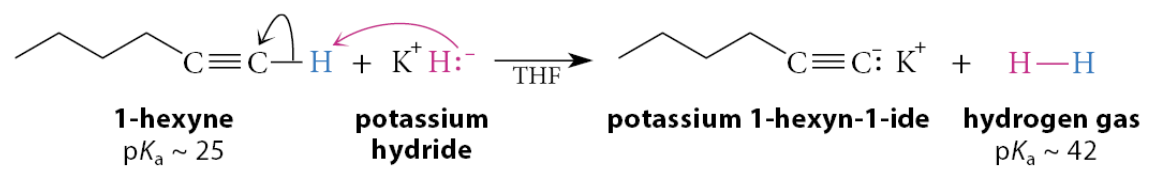
What react too slowly to be useful in the analogous reactions?
Sodium hydride (NaH) and lithium hydride (LiH)
Are acetylenic anions strong bases?
Yes, and strong nucelophiles
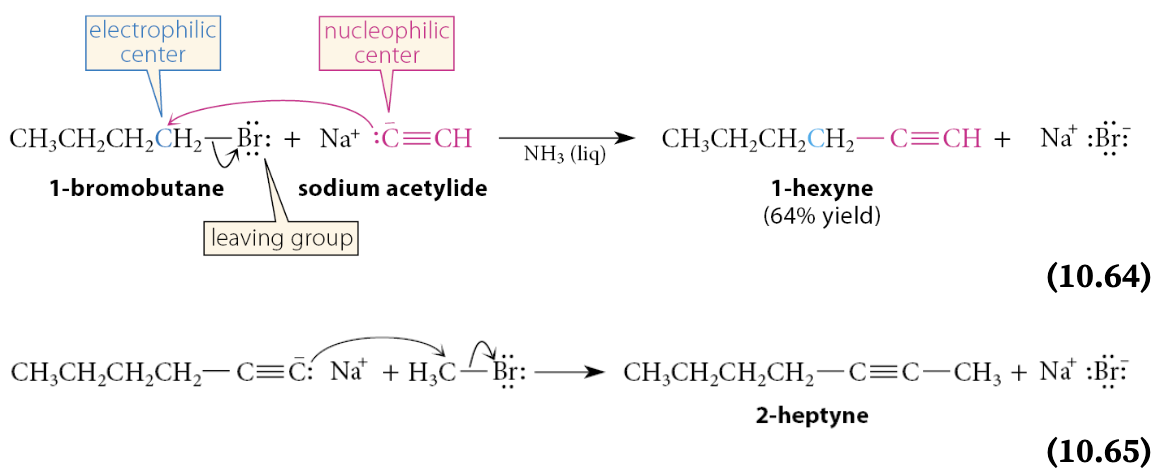
What can acetylenic anion nucleophiles be used for?
In SN2 reactions can be used to prepare other alkynes
The alkyl halides used in this reaction must be unhindered primary compounds
This reaction is important because it is a method of forming carbon–carbon bonds. Reactions such as this can be used to build up carbon chains
Do most Grignard reagents react with alkyl halides?
Most Grignard reagents do not react with alkyl halides, and the reason is the covalent character of the carbon–metal bond
10.7 Carbenes and Carbenoids
What happens when when chloroform is treated with an alkoxide base such as potassium tert-butoxide?
A small amount of its conjugate-base anion is formed
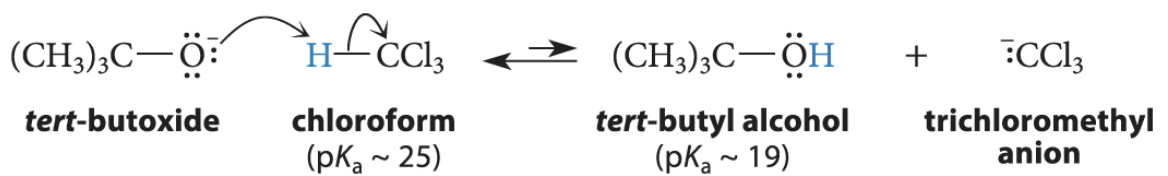
This anion can lose a chloride ion to give a neutral species called dichloromethylene
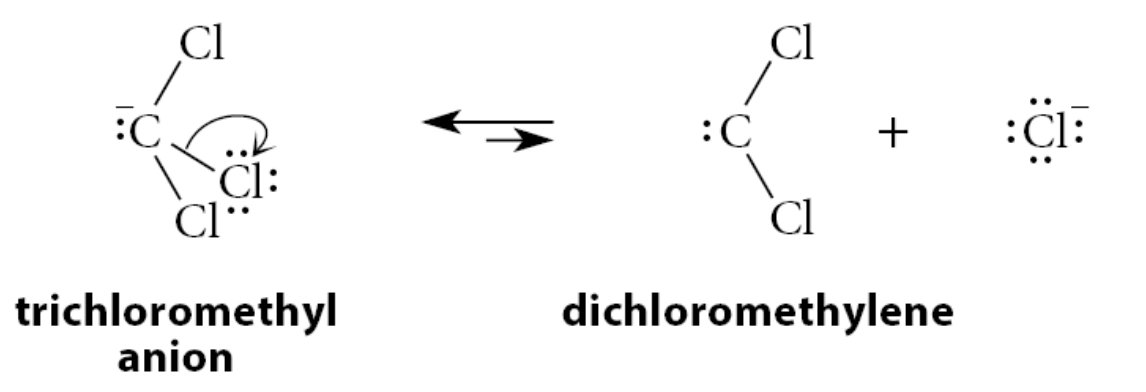
What is a carbene?
A species with a divalent carbon atom
Carbenes are unstable and highly reactive species
What is an α-elimination?
An elimination of two groups from the same atom
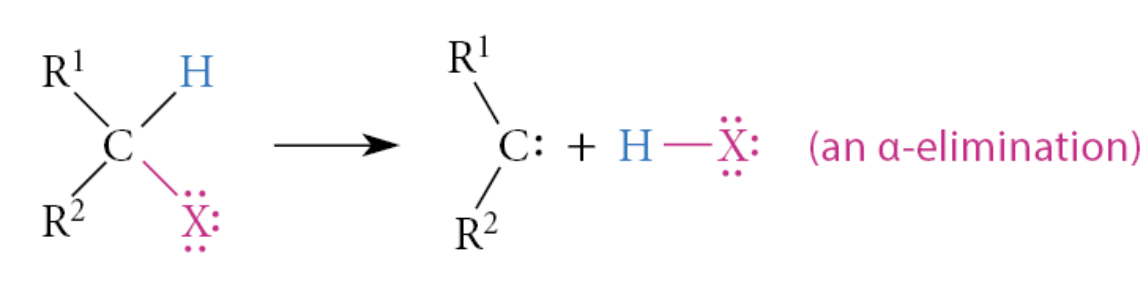
When is β-elimination preferred?
When an alkyl halide has β-hydrogens, β-elimination occurs in preference to α-elimination because alkenes, the products of β-elimination, are much more stable than carbenes, the products of α-elimination
Is dichloromethylene a powerful electrophile?
Yes, because dichloromethylene lacks an electronic octet, it is an electron-deficient compound and can accept an electron pair; therefore, dichloromethylene is a powerful electrophile
What can the divalent carbon of a carbene act as?
The divalent carbon of a carbene can act as a nucleophile and an electrophile at the same time.
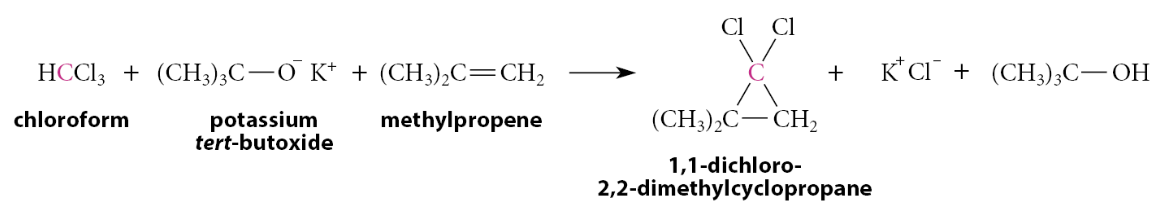
What happens when dichloromethylene is generated in the presence of an alkene?
When dichloromethylene is generated in the presence of an alkene, a cyclopropane is formed
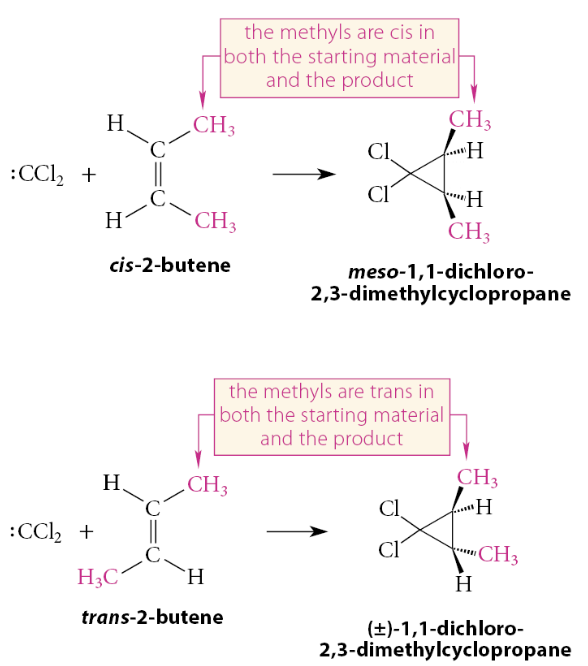
Mechanistically, the reaction is a concerted syn addition
The reaction is a stereospecific syn addition
What is the Simmons-Smith Reagent?
Iodomethylzinc iodide (I—CH2—Zn—I)
What is the stereochemistry for Simmons-Smith Reactions
Addition reactions of methylene from Simmons–Smith reagents to alkenes, like the reactions of dichloromethylene, are stereospecific syn additions
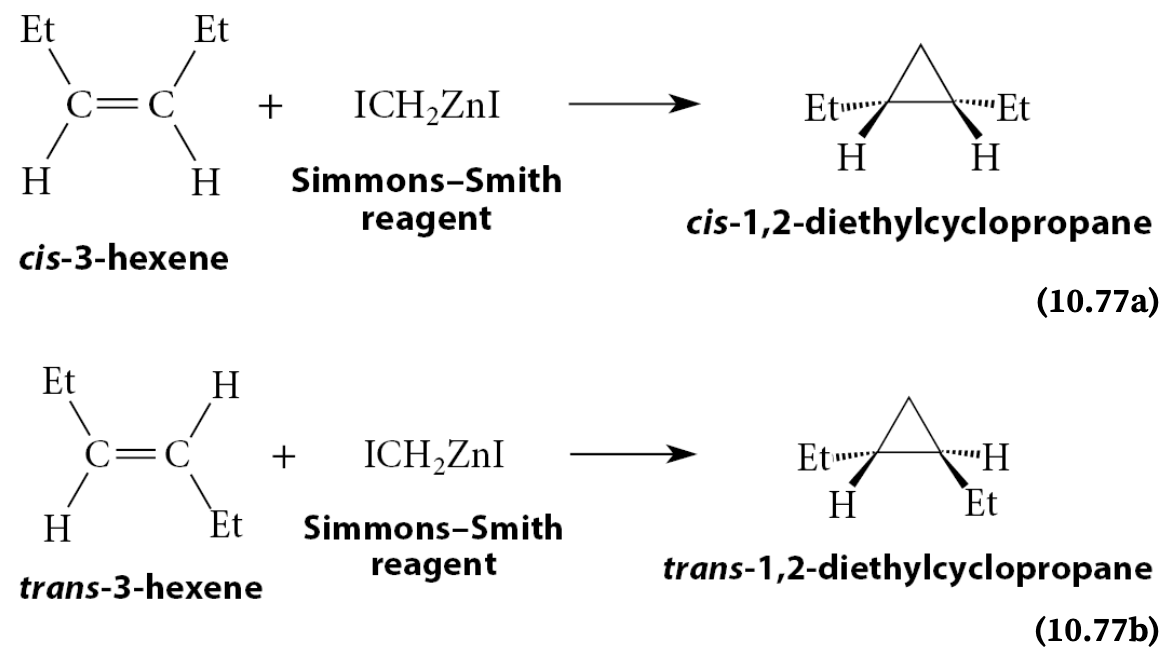
What is a carbenoid?
A reagent that is not a free carbene but has carbenelike reactivity
Because they show carbenelike reactivity, α-halo organometallic compounds are sometimes called carbenoids
What does addition of carbenes or carbenoids to alkenes yield?
Addition of carbenes or carbenoids to alkenes to yield cyclopropanes is another reaction that forms new carbon–carbon bonds