Stereoisomers
1/87
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
88 Terms
Draw examples of stereoisomers
stereoisomerism only happens with what types of molecules?
alkenes and chiral molecules
What is a chiral molecule?
A molecule which has a non-superimposable molecule as its mirror image. The carbon is sp3 hybridised and is attached to 4 different groups/substituents
What type of molecules are superimposable?
symmetrical molecules and non-chiral molecules
Are all chiral molecules non-superimposable?
yes
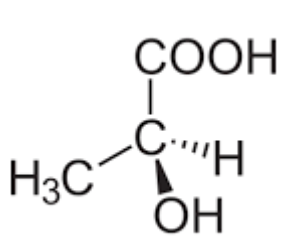
Draw the mirror image of this molecule
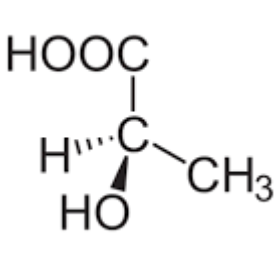
Are stereoisomers the same compound?
completely different compound
Does DNA have a chiral centre? What about a funnel?
-yes. ALL spirals are chiral
-no
What are enantiomers?
-mirror image isomers (chiral molecule and its mirror image). They are non-superimposable and come in pairs
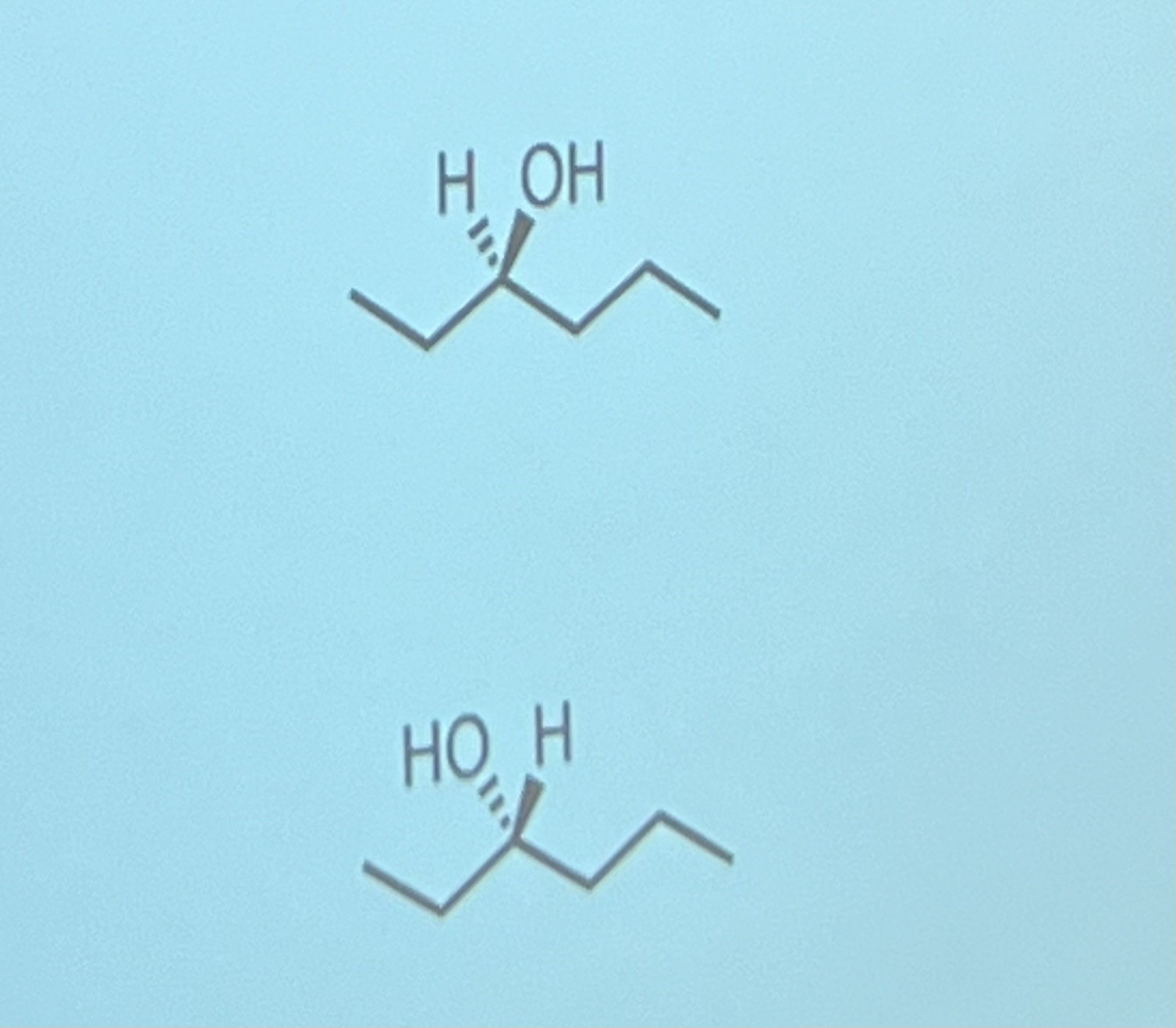
Draw the enantiomers of hexan-3-ol
you only show the bonds involved in the chirality
Is this molecule chiral?
achiral as it is symmetrical
If a molecule has a lone pair, is it basic or acidic?
basic
What is a hydride ion?
H-
How many bonds does nitrogen need to make to have a full shell?
so lone pair always present unless something like NH4+
enantiomers are either… or …
R or S
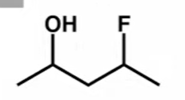
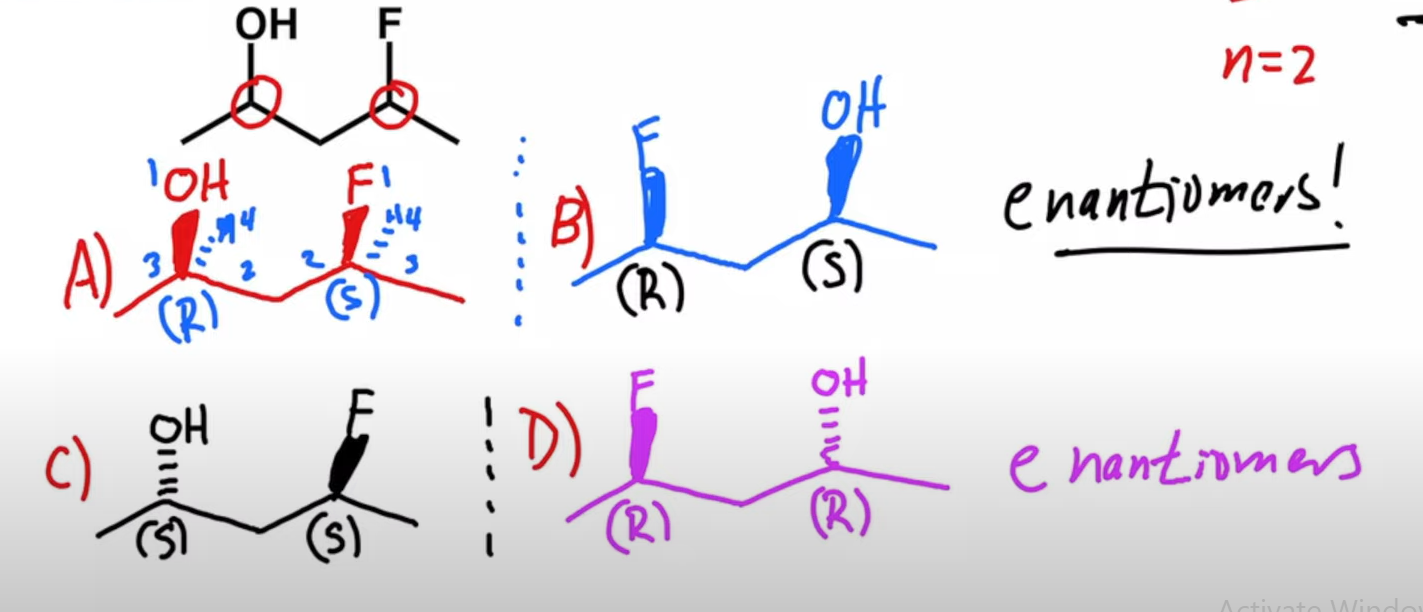
Draw all the possible stereoisomers of this molecule
A= original molecule
B=reflection of the original molecule
C=changed 1 wedge from original molecule into its opposite wedge
D=mirror image of C
THIS APPLIES TO ALL MOLECULES WITH MORE THAN ONE CHIRAL CENTRE
To draw the diastereoisomer of a molecule with 2 or more chiral centres, do a single opposite wedge change on at least 1 chiral centre, not all.
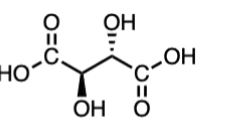
What type of molecule is this?
meso compound
-even if we draw the ‘enantiomers’ of the compound, it is still an enantiomer
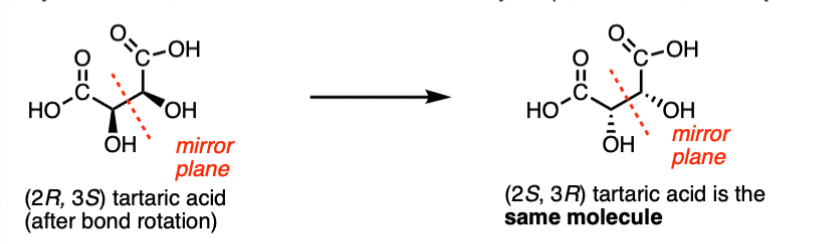
Rules for drawing enantiomers (e.g. 1s to 1r or 1,s,2s to 1r,2r)
-swapping groups changes configuration to opposite
-swapping the wedges changes configuration to opposite
-if the molecule has single wedge, draw opposite wedge for enantiomer
These rules apply to molecules with 2+ chiral centres
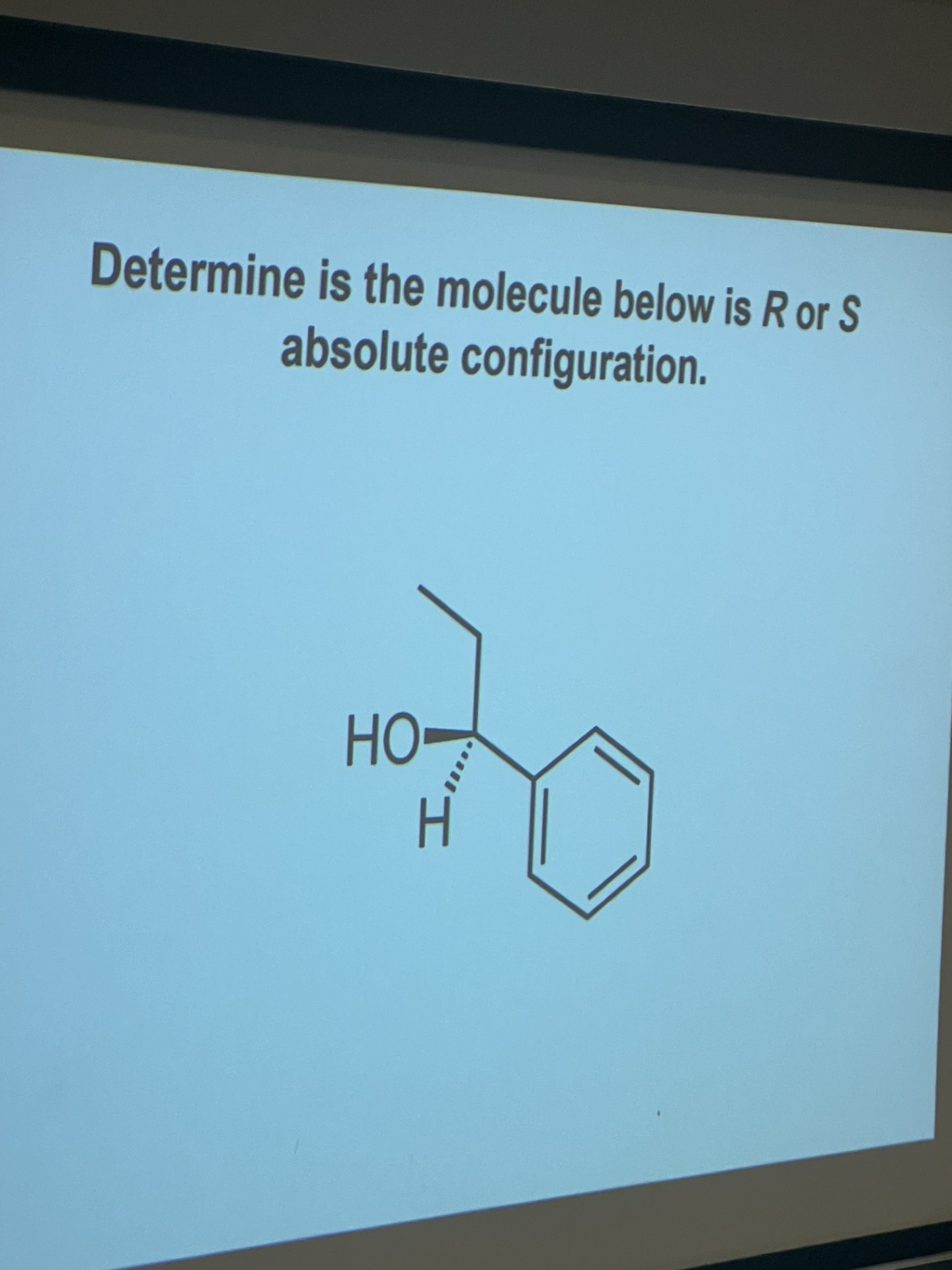
Explain how to determine the configuration of enantiomers
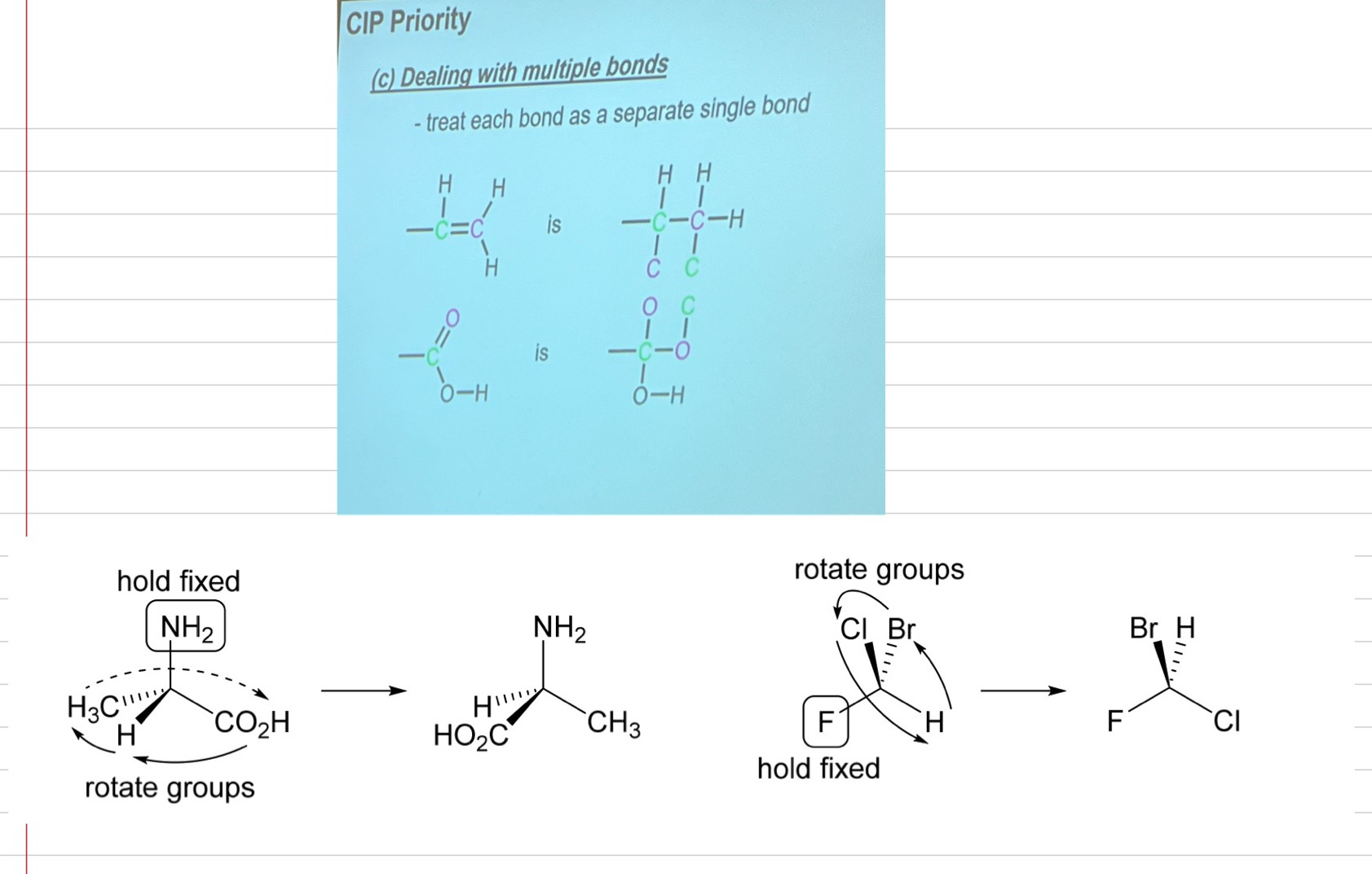
identify the 4 different substituents bonded to the chirality centre and number them based on priority (atomic number NOT atomic mass). The atoms do NOT add up!! If there are multiple bonds do what it shows in image
draw the molecule with the lowest priority group behind the plane of the paper. If the structure doesn’t have the lowest priority behind the plane of the paper, do a simple rotation (as shown in image).
Now number the priorities. clockwise= R (R for right). S=anti-clockwise. It’s enantiomer will be the opposite. So, one enantiomer will be R and the other will be S. Never switch the bonds, just the molecules
cheat code:
if the lowest priority is at the FRONT (bold wedge), number priorities and whatever configuration you get, the answer is the opposite. e.g. if you get S, the answer is R. BUT YOU MUST MAKE THIS CLEAR IN EXAM AS TEACHER THINKS YOU ARE GUESSING
Draw other groups completely
What properties of enantiomers are identical?
-physical e.g. desnity, mp, bp, polarity, solubility EXCLUDING interaction with plane-polarised light
-chemical properties e.g. pKa EXCLUDING when they react with other chiral molecules
What property of enantiomers is different?
pharmacological
Is plane-polarised light only unique to chiral molecules?
-yes. Therefore, chiral molecules are optically active
Explain plane-polarised light for chiral molecules
-If a substance causes the plane of polarised light to rotate clockwise, a (angle) is positive (+) and the substance is called dextrorotatory. Anti-clockwise, a has a negative value (-) and the substance is called levorotatory
What is the equation for calculating the specific angle of rotation for chiral molecules?
c=concentration (% or g/100mL)
l= path length that the light travels through (dm) (to convert from cm to dm, you divide by 10)
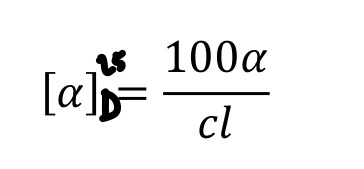
State the similarity and/or differences in enantiomers when it comes to their magnitude and direction
-the specific rotations for a pair of enantiomers are EQUAL in magnitude but opposite in direction. e.g. S-lactic acid= +33.3 degrees R-lactic acid=-33.3% THERE IS NO RELATIONSHIP BETWEEN ABSOLUTE STEREOCHEMISTRY (R AND S) AND OPTICAL ROTATION
Why do enantiomers have different pharmacological properties from each other? Why is this important?
-because proteins are also chiral. So a complementary drug must bind to it. Only one of the enantiomers will be complementary.
-sometimes, a drug is manufactured as a mixture of both its enantiomers. One enantiomer must be active and the other inactive. The inactive one should do nothing, BUT it may contribute to adverse drug reactions or toxicity. It’s not good to administer the inactive substance for this reason.
How do you draw the enantiomer of a molecule?
-mirror image
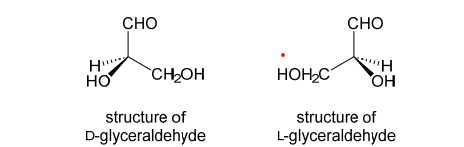
Explain D and L in terms of configuration. Draw any relevant structures
The configuration of substituents found in (+)-glyceraldehyde was called the ‘D’ configuration. The configuration of substituents found in (-)-glyceraldehyde was called the ‘L’ configuration
Compounds that were chemically similar to D-glyceraldehyde, with the same arrangement of bonds, were also assigned the D configuration (irrespective of their optical rotation). Similarly, compounds related to L-glyceraldehyde were assigned the L configuration. D and L can really only be sensibly used for compounds that are chemically related to glyceraldehyde. All of the amino acids found in proteins (those coded for by the genetic code) are L relative configuration. Similarly, all of the common carbohydrates found in nature are D relative configuration.
If a molecule has 2 stereoisomers, how many enantiomers does it have?
4
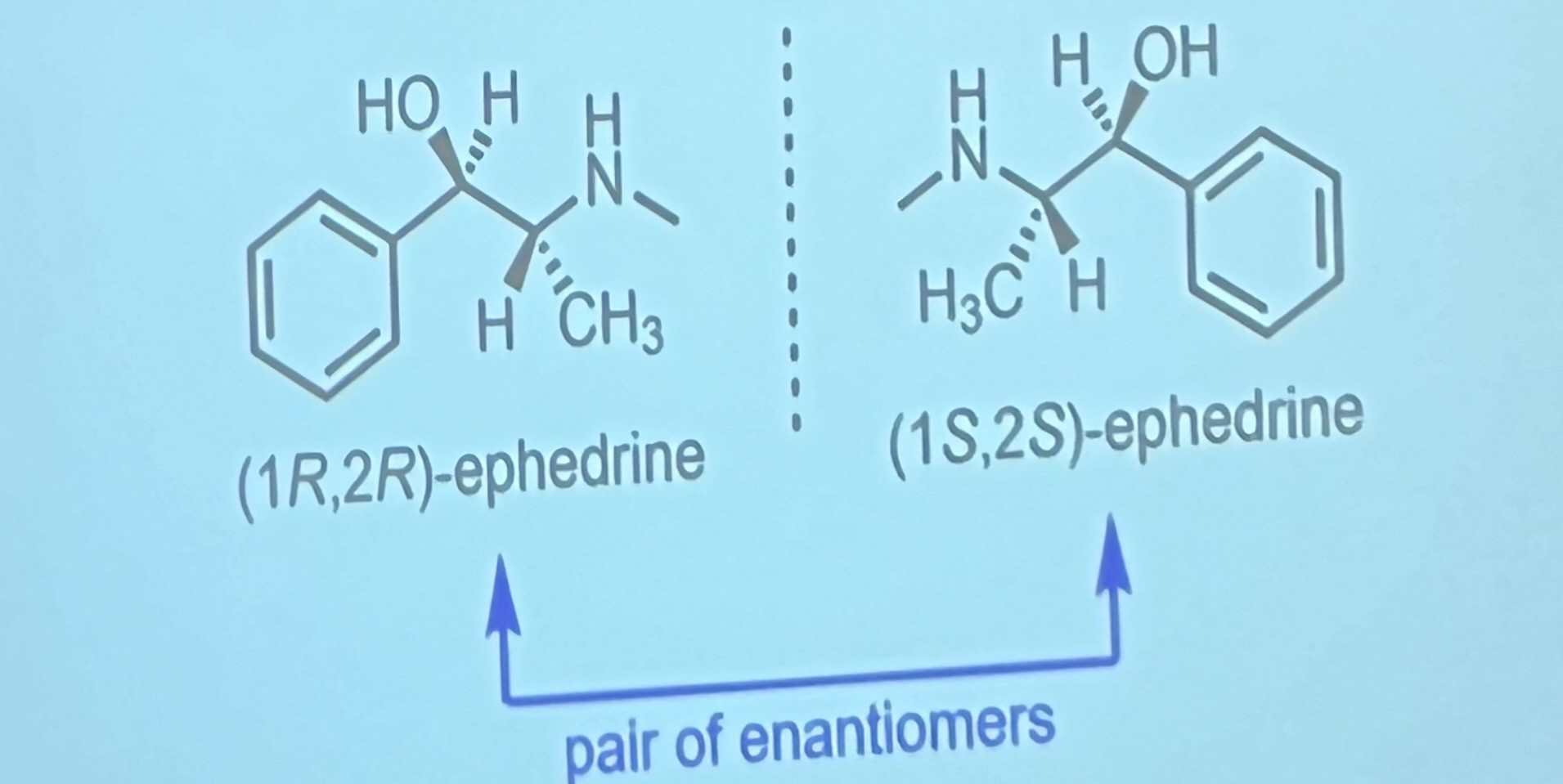
What is an enantiomer?
non-superimposable mirror image isomers that have opposite configuration at EVERY chirality centre
s
What is a diastereoisomer?
stereoisomers that are NOT enantiomers (not the mirror image basically)
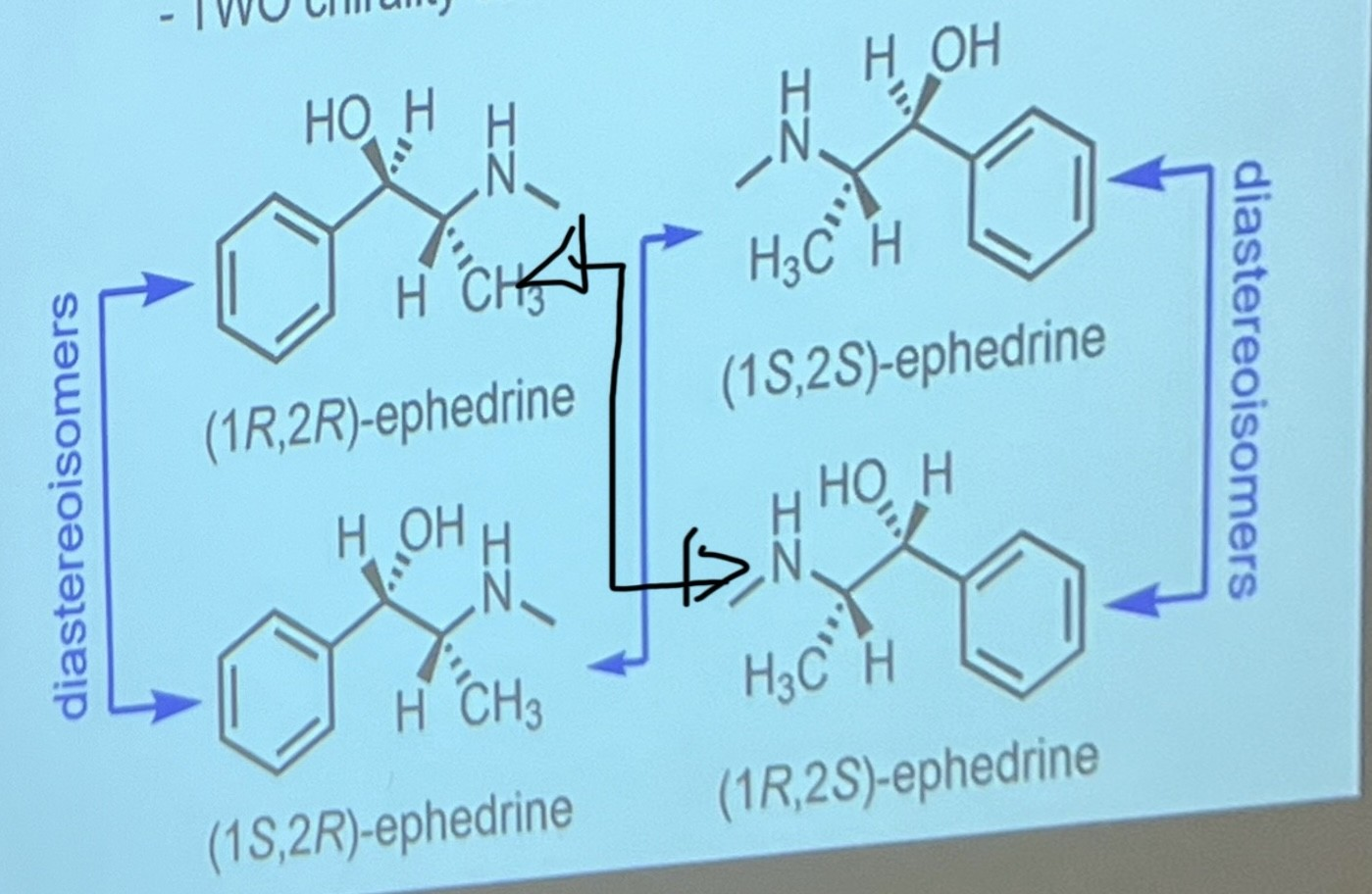
State the properties of diastereoisomers
They are completely different compounds to each other. Therefore, they have different mp and bp, polarities, solubilities, pharmacological activities etc.
Are diastereoisomers optically active?
yes because it is still chiral
What is the general rule for the number of stereoisomers in a molecule?
2^n stereoisomers (n=number of chiral centres)
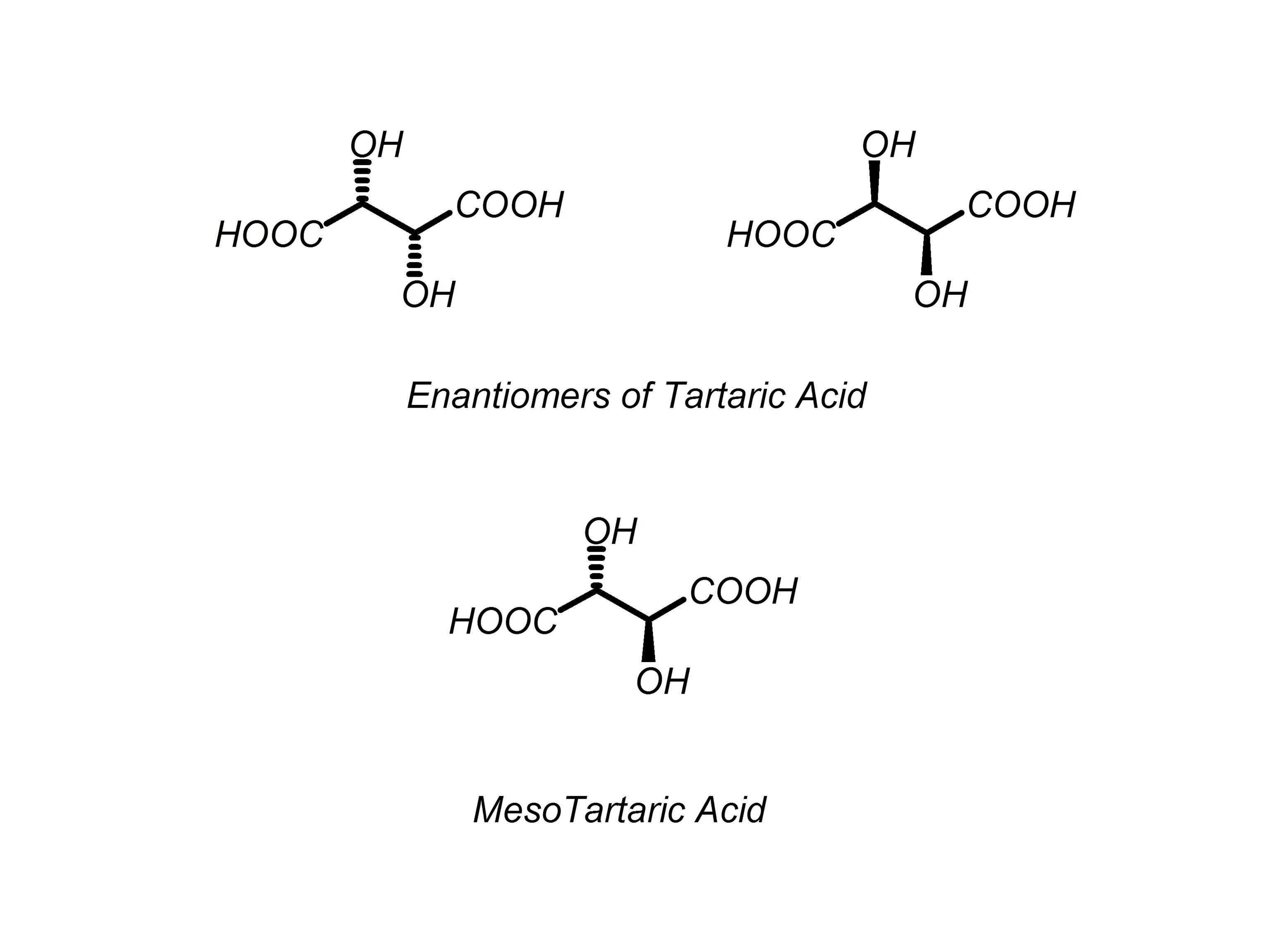
What are meso compounds?
-compounds that have 1 or more stereoisomers that have a plane of symmetry and are thus achiral.
-achiral=optically inactive
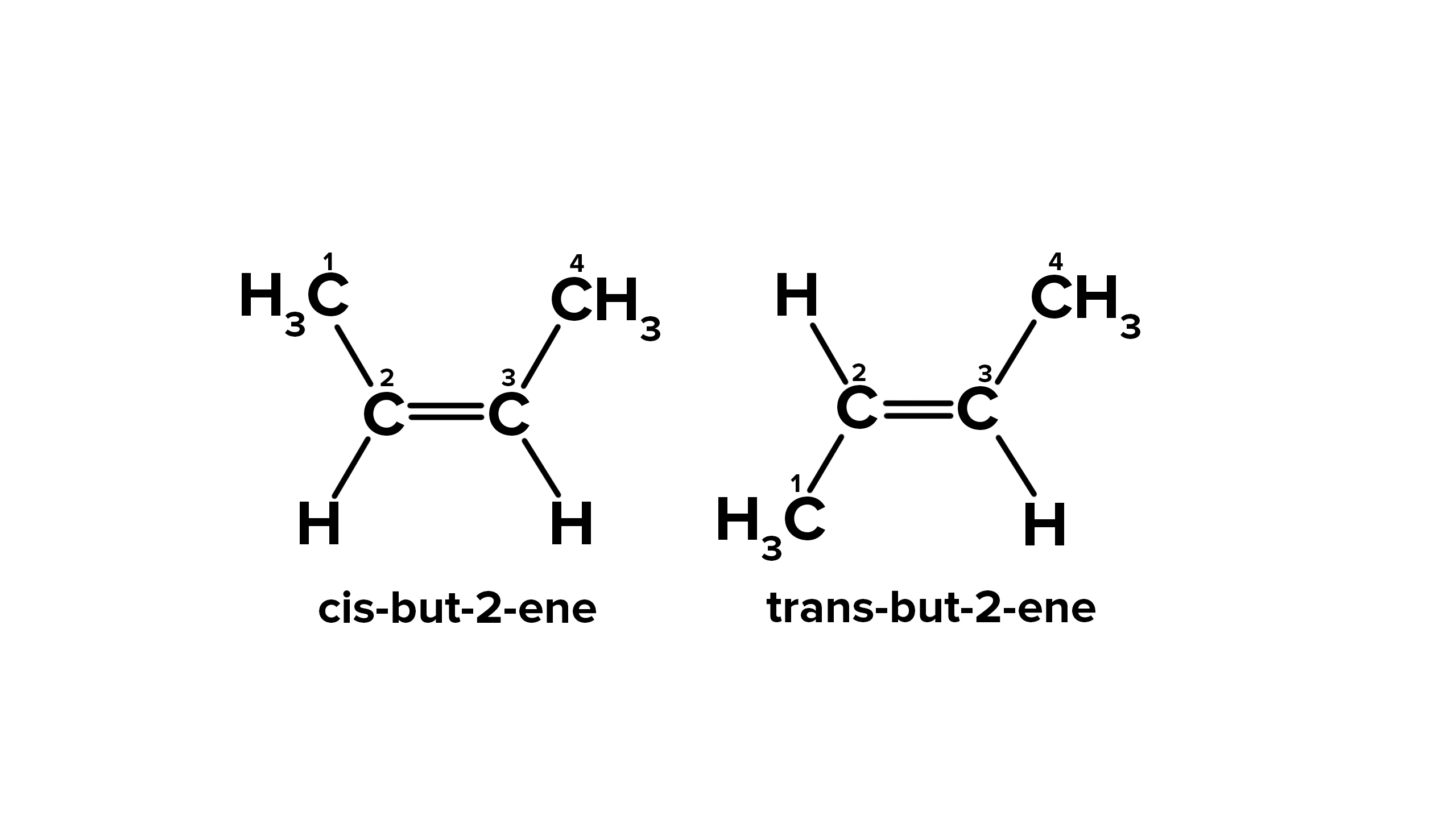
When is cis-trans naming done?
-each sp2 carbon is bonded to a H and one other group
-non hydrogen groups on same side= cis
-non-hydrogen groups on different sides=trans
Name this molecule using cis-trans
cis-but-2-ene
State the physical, chemical and pharmacological activities of cis-trans isomers
-completely different as they aren’t the same molecule
What type of isomers are cis-trans isomers?
diastereoisomers because they are achiral, superimposable . They are stereoisomers that are NOT mirror images of each other (e.g. cis-but-2-ene and trans-but-2-ene
What is used if cis-trans can’t be used?
E/Z
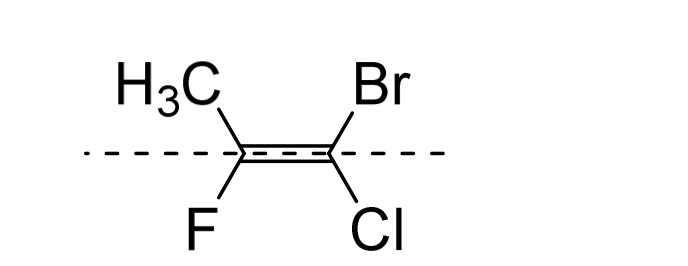
Name this molecule using CIP
E-1-bromo-1-chloro-2-fluoroprop-1-ene
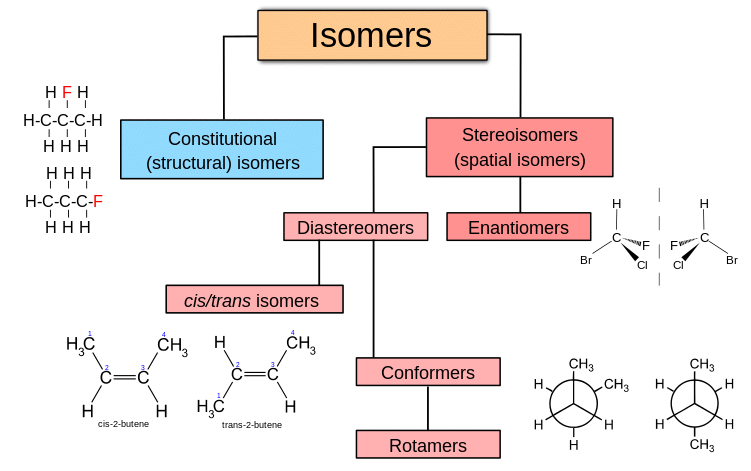
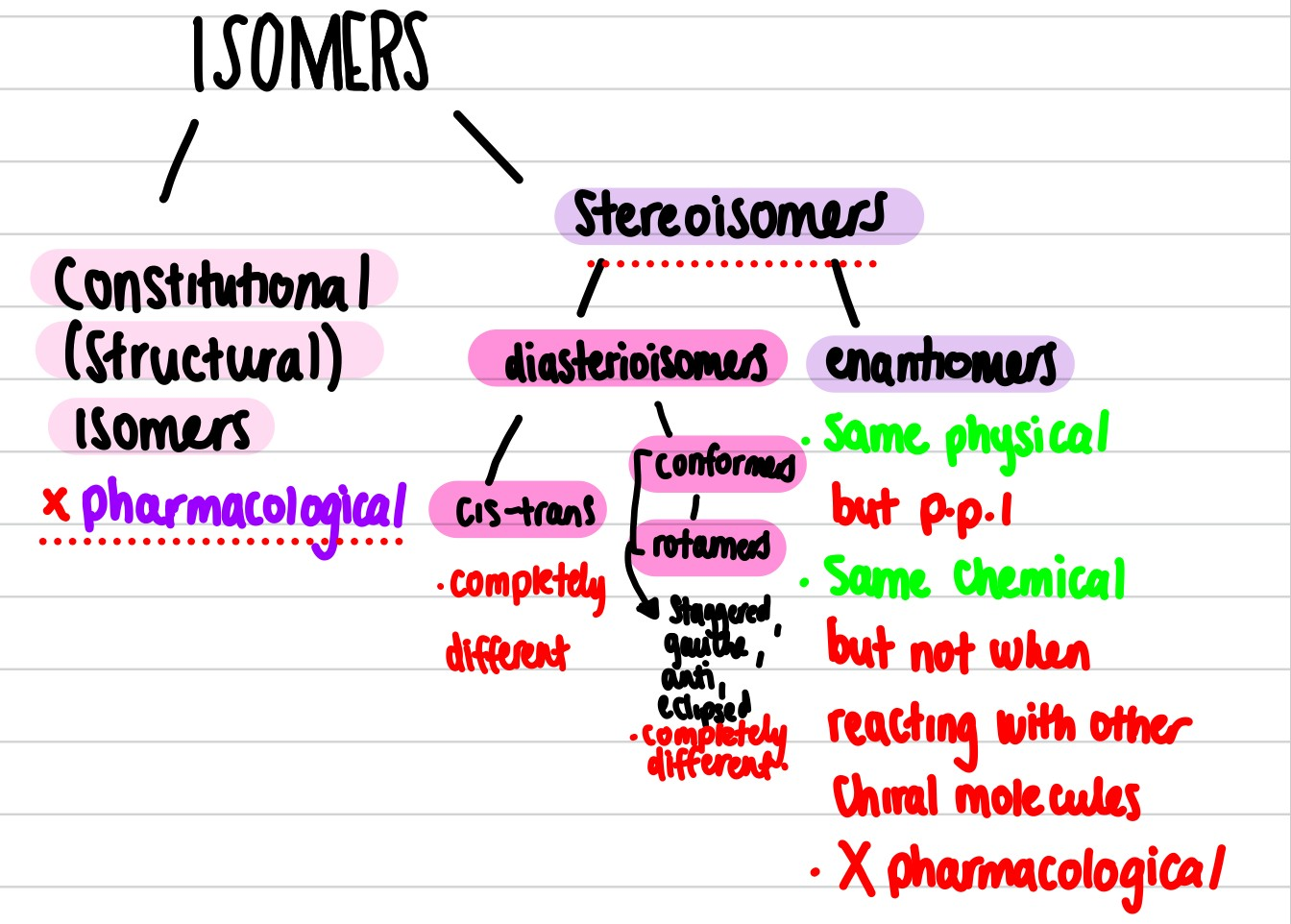
Draw the isomerism tree
What does retention of configuration mean?
when a reaction takes place in the part of the molecule that isn’t the chirality centre, so the molecule’s configuration will be the same after the reaction
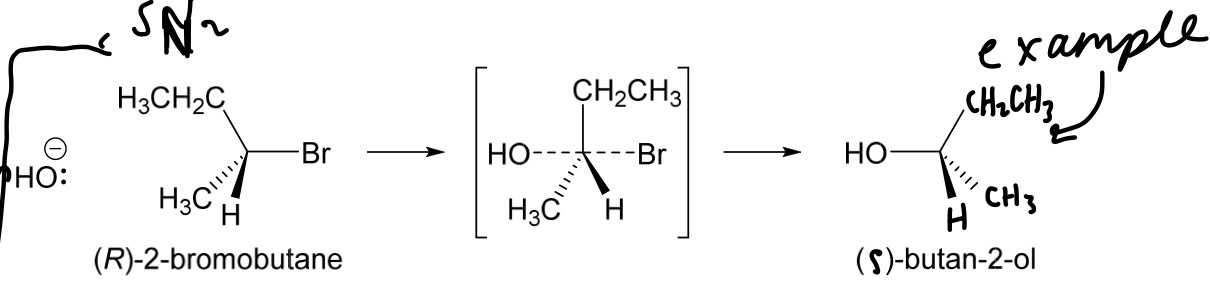
What happens to chiral molecules when they react via SN2 mechanism/reaction?
-inversion of configuration
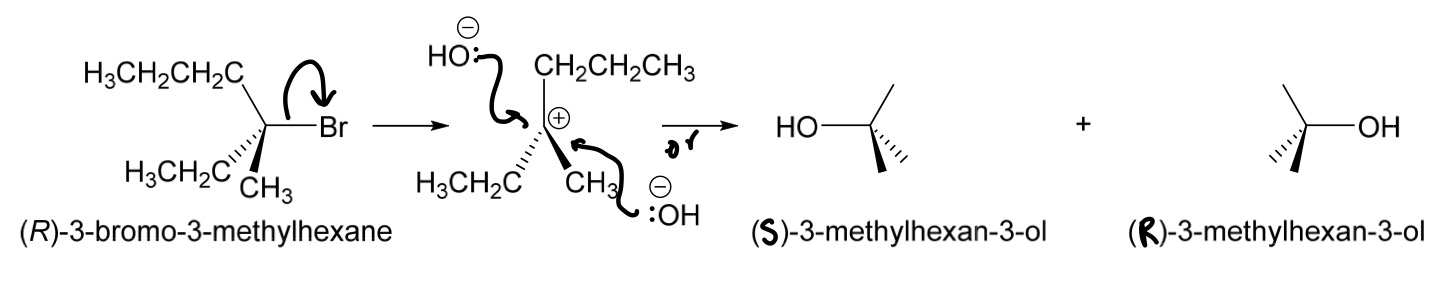
What happens to chiral molecules when they react via SN1 mechanism/reaction? How would the mechanism look like?
racemisation/racemic mixture (a 1:1 mixture of enantiomers. No optical activity)
-for enantiomers, one is always S and the other is R
How are the enantiomers in a a racemic mixture separated?
resolution. Very hard as enantiomers have the same reactivity
What are the main drug targets?
receptors, where they either act as an agonist or antagonist
enzyme
there are other drug targets too e.g. some drugs bind to DNA directly
Why can’t enantiomers bind to stereoisomer’s binding site?
Every target site, including DNA, has a 3D binding site. Only drug molecules with complementary fitare able to bind to drug. (e.g. right foot= left shoe x)
Do enantiomers get absorbed in the same way?
yes because they have the same polarity
How do you draw enantiomers when it’s a big molecule?
literally just swap the relevant molecules NOT the wedges (or swap the wedges, not th groups). don’t need to reflect whole molecule.
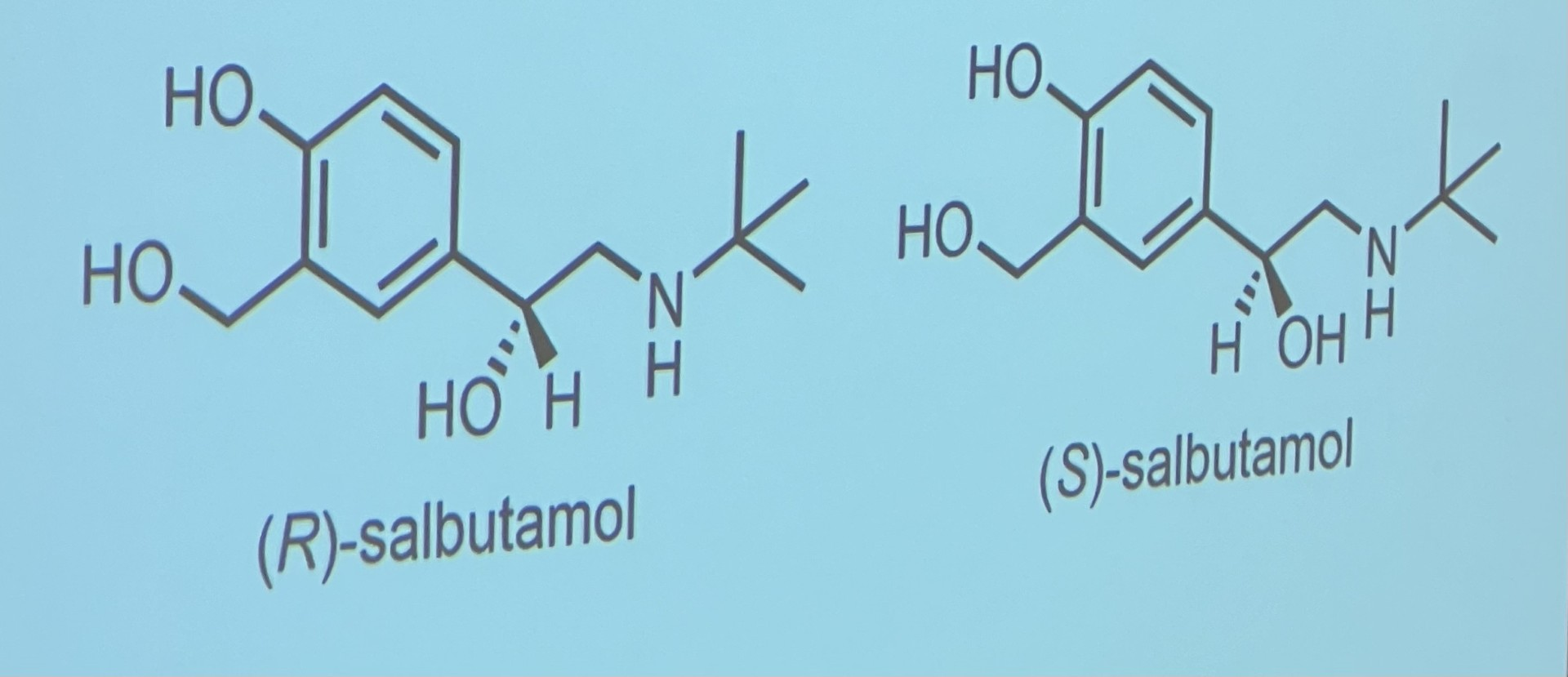
Are enantiomers metabolised in the same way?
no because they have different pharmacological properties
Can a drug bind to a receptor if the drug is R configuration and the receptor is S configuration?
yh
Do isomers have the same molecular formula?
yes
Can you do E-Z for cis-trans?
yes but you CANNOT do cis trans for e z
-(E for everything)
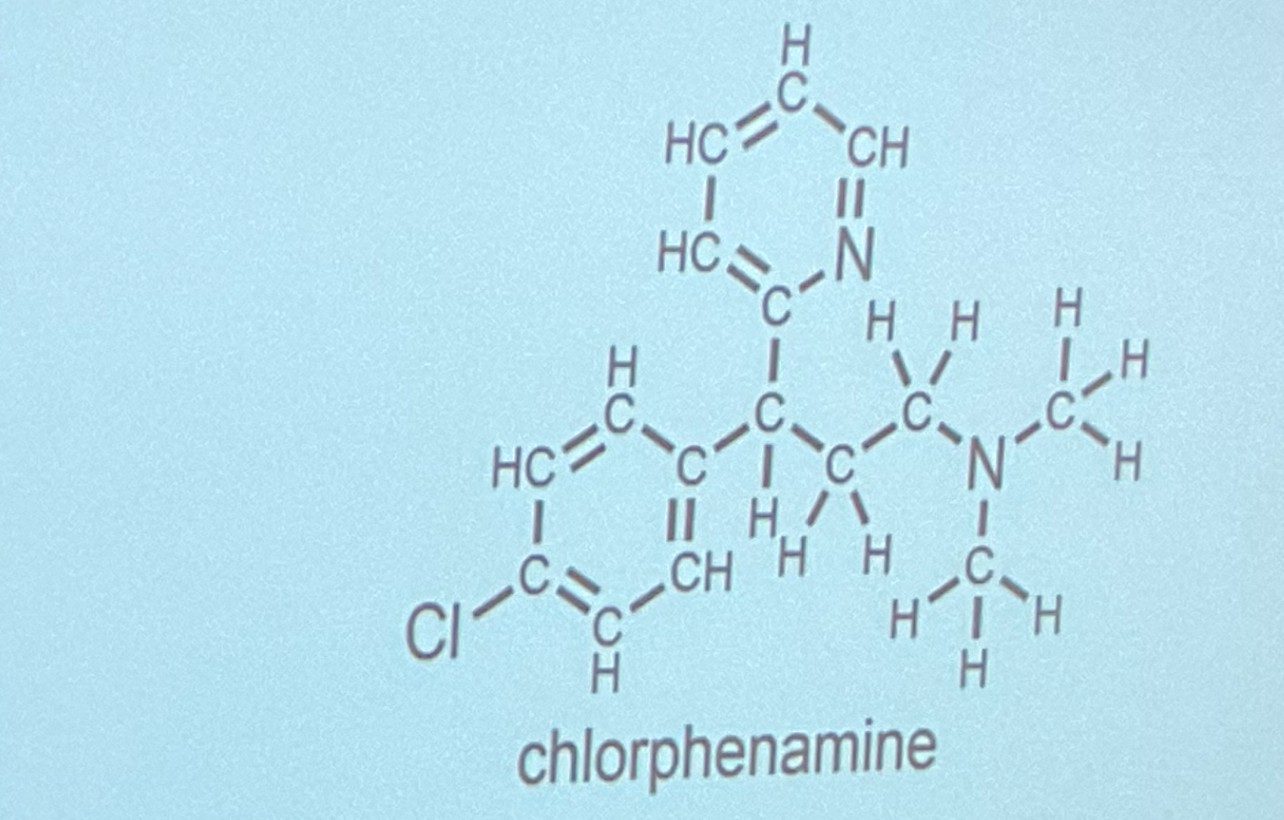
Can BOTH N atoms do hydrogen bonding?
yes. The N’s can form a hydrogen bond because of 1 lone pair on each of them and turn into positive nitrogens
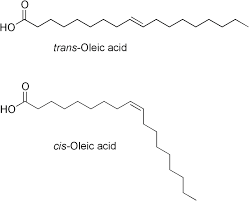
What type of unsaturated fatty acids have a higher melting point, cis or trans fatty acids?
trans. solid at rt likely. linear, so pack closely
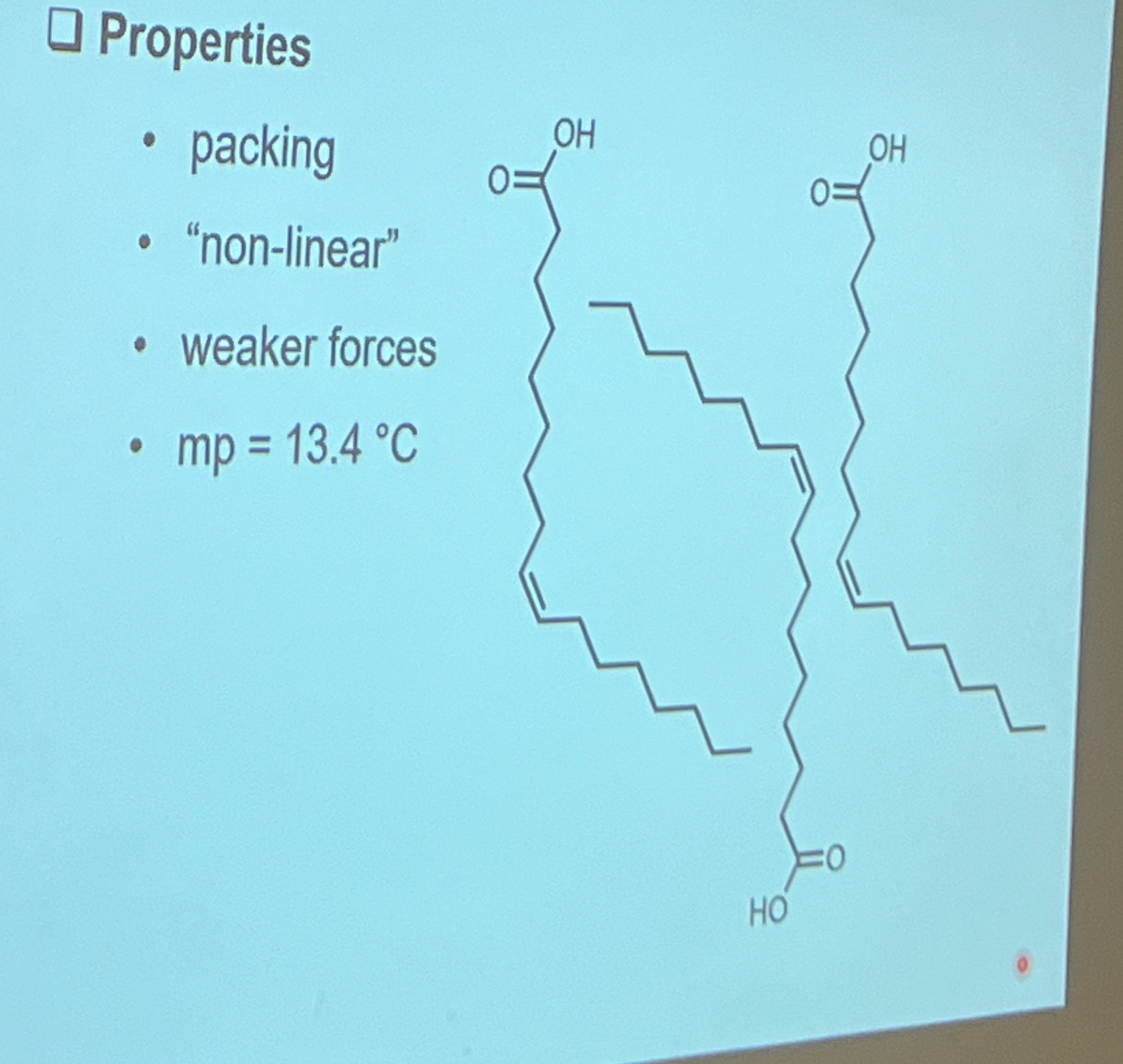
Why do cis and trans fatty acids have different pharmacological properties?
-they are diastereoisomers, so they bind to different target sites and therefore have different pharmacological properties
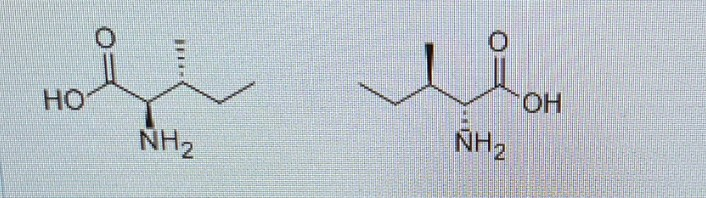
Why are these not enantiomers?
ALL chiral centres MUST be mirror images of each other in order for it to be enantiomers of each other
CIP rules
find the first difference in the molecule, including H bonds.
the atom directly bonded to the double bonded carbons, you check which has higher atomic number
If the first atom on both substituents are the identical, then proceed along both substituent chains until the first point of difference is determined.
remember that atoms involved in multiple bonds are considered with a specific set of rules. These atoms are treated as if they have the same number of single-bond atoms as they have attached to multiply bonded atoms.
Which has a higher atomic number, N or O?
O
How to do R and S configuration when the lowest priority is in the plane of the molecule instead of the back
Swapping any two groups on a chiral center inverts its absolute configuration (R to S, S to R):
The lowest priority group is in the drawing plane, so what we can do is swap it with the one that is pointing away from us
The arrow goes counterclockwise indicating S configuration and this means in the original molecule it is R.
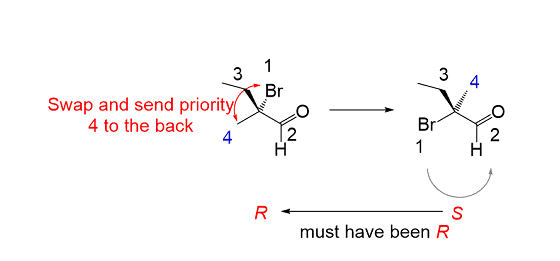
What to do if the lowest priority is in the plane of the paper and there is no dashed wedge present OR if the lowest priority group isn’t shown at all (because it is H)?
nothing. Whatever the configuration is, keep it the same
-there must always be 2 normal bonds, a striped wedge and bold wedge ALWAYS. so if one isn’t present, then you can figure out what bond it is
When figuring out priority group in stereoisomers, always look for…
highest atomic number first and then look for THE FIRST DIFFERENCE

(a) Identical; (b) Enantiomers.
remember switch configuration whenever the lowest priority isn’t at the back (even if it’s not H)

Clavulanic acid is R,R,Z

+54°

Inactive enantiomer may contribute to unwanted effects; synthesis/isolation of single enantiomer may be difficult and costly.
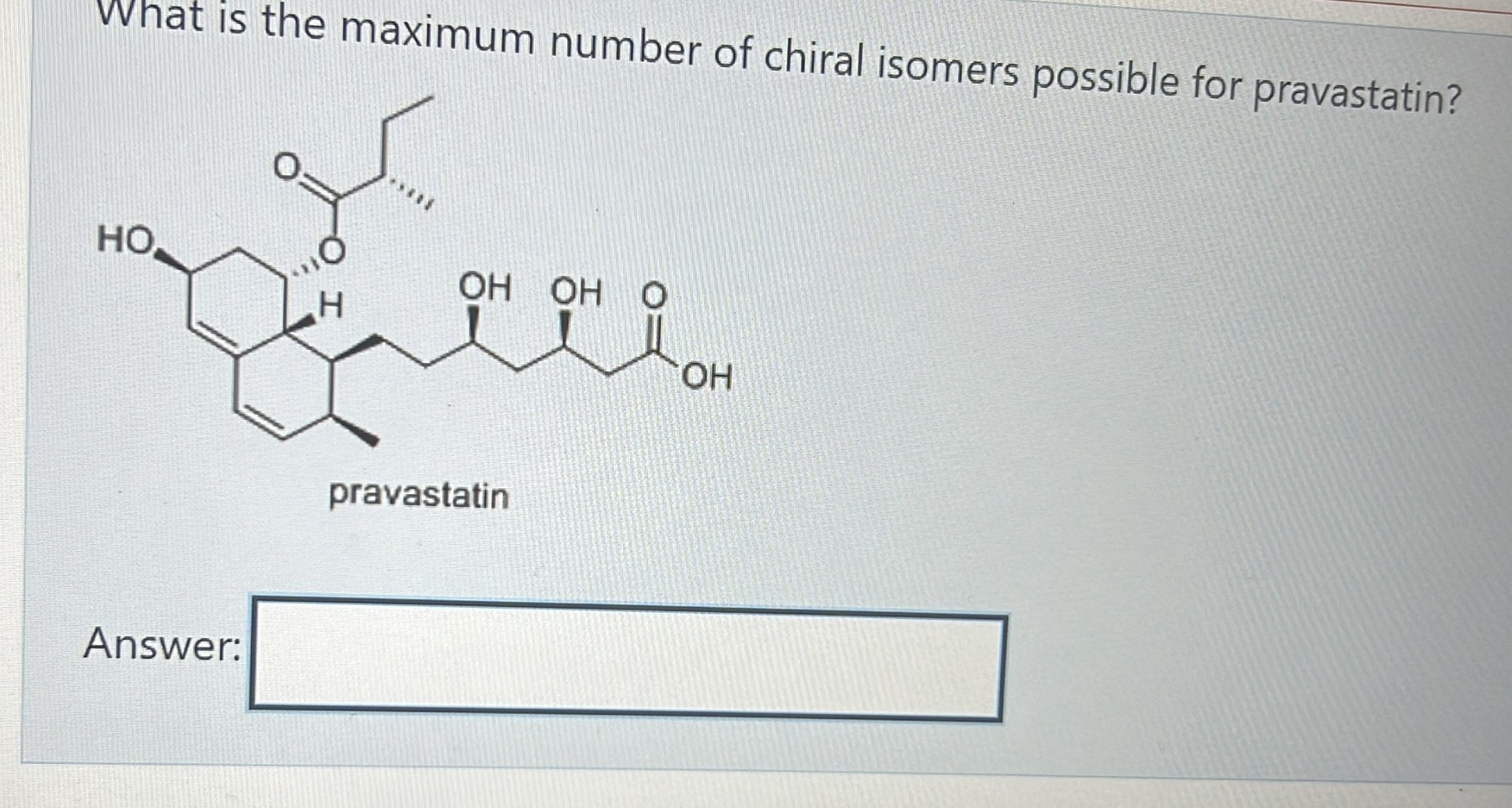
256
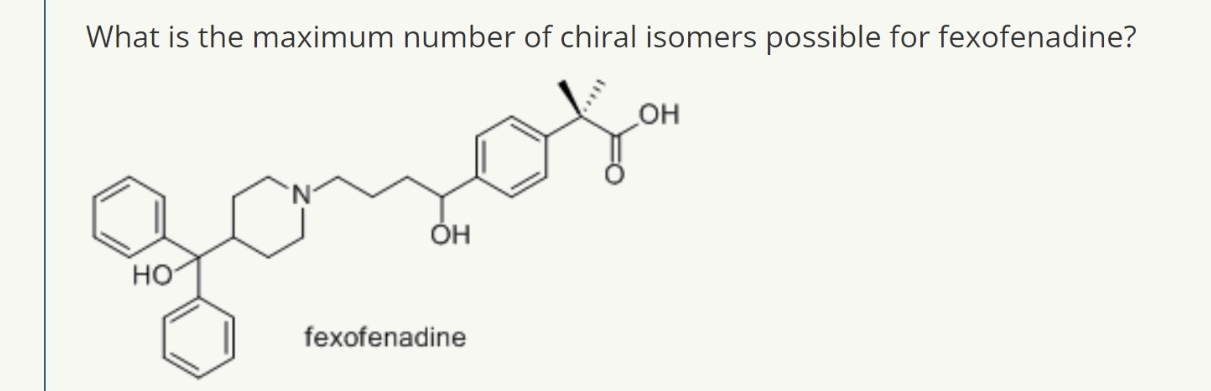
1
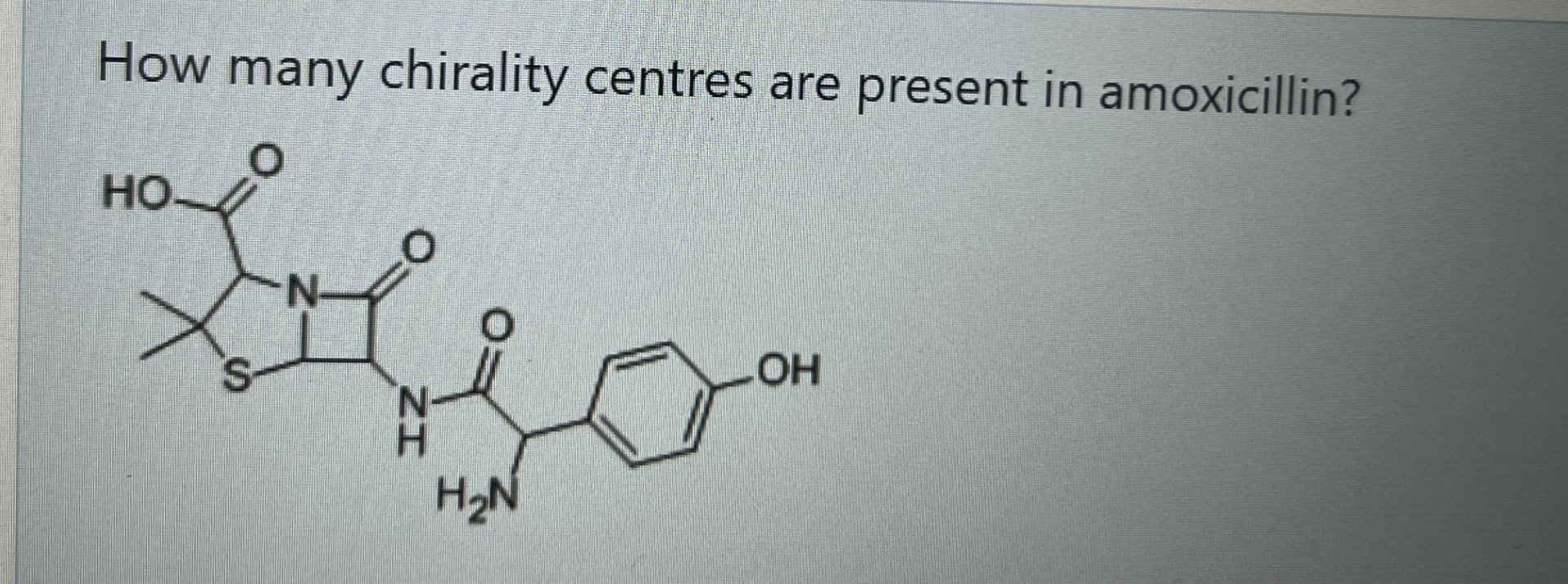
4

R
REMEMBER THE DOUBLE BOND RULE
All the physical properties of two enantiomers are identical. True or false?
False. They have completely identical properties APART FROM THEIR OPTICAL ROTATION. They rotate plane polarised light by equal amounts in opposite directions
A racemic mixture is a 1:1 mixture of any two stereoisomers. True or false?
false. 2 enantiomers specifically
Any single chiral molecule only has one enantiomer. True or false.
True (enantiomers come in pairs)
Do a pair of cis-trans isomers have the same biological activity?
no e.g. our bodies can process cis fatty acids but not trans
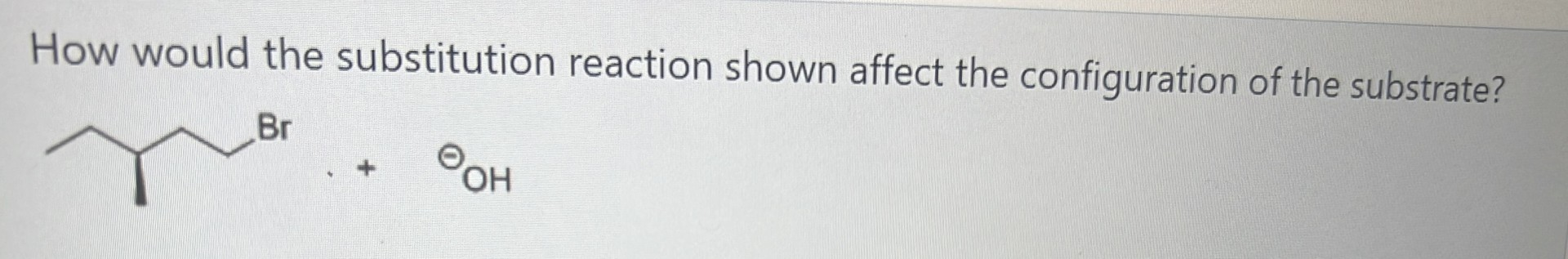
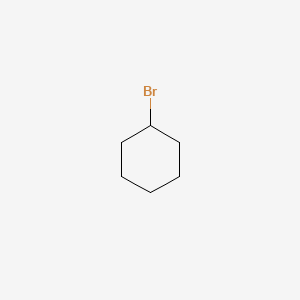
-retention of configuration
-not inversion because the C attached to the Br is not a chiral centre.
-not racemisation because this is not a tertiary alkyl halide
-substrate/product is a chiral molecule itself, so can’t be ‘‘substrate/product is achiral’’
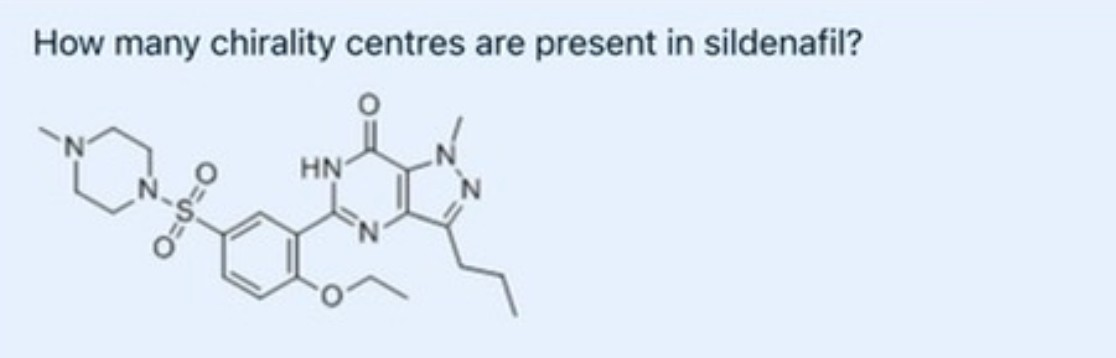
0
Do cis-trans isomers have the same biological activity?
no e.g. cis fatty acids can be processed by our enzymes in our body but can’t break down trans fatty acids as our body hasn’t got the sufficient enzymes required.
Isomers labelled tree
cis-trans different on everything

16.2

4mg

One positive and two negative.
Cis-fatty acids are more stable than trans fatty acids. True or false? Why?
false
-Cis fatty acids have a kink or bend in the hydrocarbon chain due to the hydrogen atoms being on the same side of the double bond. This makes the molecule more fluid and less tightly packed, which leads to:
Lower melting points
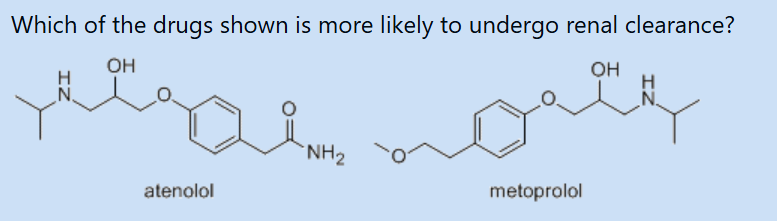
atenolol
What does oxidising a molecule mean?
it loses electrons, gains oxygen, or loses hydrogen.
What does rounding to the nearest 0.1% mean?
rounding to 1 dp