Lecture 19 - bioenergetics
1/41
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
42 Terms
how do cells conserve energy in order to drive as many reactions as possible
To do this, the biological reactions occurs in multiple enzyme- catalyzed steps (a pathway) mostly with small free energy changes
why do cells use multistep pathways?
Instead of releasing all that energy in one step (which would be wasteful and uncontrollable), cells:
Use multiple enzyme-catalyzed steps organized in metabolic pathways.
Each step generally has a small ΔG°', meaning only a small amount of energy is released at a time.
Some steps do have larger ΔG°' values where energy is captured and stored.
what is this captured energy stored as?
This captured energy is stored in "quanta" — small packets, commonly in molecules like:
ATP
NADH
FADH₂
what are types of Biological Energy Quanta? (2)
Reduced electron carriers:
Nucleoside triphosphates:
what are Reduced electron carriers?
Examples: NADH, NADPH, FADH₂, FMNH₂
Function: Store energy temporarily by carrying electrons; later used in oxidative phosphorylation or biosynthesis.
what are Nucleoside triphosphates:
Examples: ATP, GTP
Function: Universal energy currency for cellular work like muscle contraction, transport, biosynthesis, etc.
function of quanta
Energy isn't released all at once.
Instead, it's captured in small, transferable packets (quanta).
These can be used across various metabolic pathways to fuel cellular work (e.g. biosynthesis, ion transport).
Role of Enzymes in favourable reactions
Even spontaneous reactions (ΔG°′ < 0) may not occur without enzymes.
Enzymes provide the mechanism (i.e., lower activation energy, stabilize the transition state) so the reaction can proceed at a useful rate.
Even if a reaction is energetically favorable, it won’t proceed without a mechanism—that’s the enzyme’s job.
where does most energy come from in biological systems?
from Oxidation-Reduction (Redox) Reactions
what are Oxidation-Reduction (Redox) Reactions:?
Involve electron transfer.
Oxidation = loss of electrons (increased oxidation state).
Reduction = gain of electrons (decreased oxidation state).
Energy is derived because moving electrons from one molecule to another can release energy.
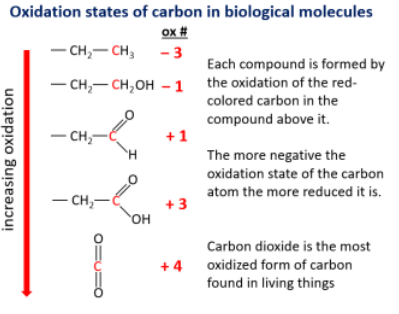
Why Carbon is Central for these reactions?
Carbon can exist in multiple oxidation states, from −3 to +4.
The more oxidized the carbon, the less energy it can release.
The more reduced the carbon, the more energy it stores (used in fats, sugars, etc.).
Examples of Oxidation States:
(Each red carbon shows the increasing oxidation state):
CH₃–CH₃ (−3): highly reduced, lots of energy.
CH₂–CH₂OH (−1): partially oxidized.
CH₂–COOH (+1 to +3): oxidized.
CO₂ (+4): fully oxidized, no usable energy left.
what is reduction?
gain of electrons
what is oxidation?
loss of electrons
what happens to the electron donor?
becomes oxidized.
what happens to the electron acceptor?
becomes reduced.
what must you need inorder for a redox reaction to occur
A redox reaction can only occur if there is both:
A donor (a reduced species with electrons to give)
An acceptor (an oxidized species that can accept electrons)
Two reducing species cant work because No one has electrons to give → no reaction. and neither can two oxidizing species because Both have electrons → no one to accept them → no reaction.
how do we know the direction of electron flow?
The direction will be determined by the relative affinity of the chemical species for electrons. Electrons will flow from molecules of low affinity to high affinity.
how do Comparing Other Molecules for electron affinities?:
The reaction H⁺ + e⁻ → ½ H₂ is like the "0 point on a redox ruler".
All other redox potentials (electron affinities) are measured relative to it.
So, the proton forming hydrogen gas gives us a universal benchmark for understanding who gives and who takes electrons in biochemical systems.

what is E° and how can you determine it?
affinity for electrons
can be determined for biological molecules (relative to the half reaction of hydrogen which equals 0) by combining the various species together and measuring the direction of electron flow.
Higher E° = greater tendency to be reduced
Lower E° = greater tendency to donate electrons (be oxidized)
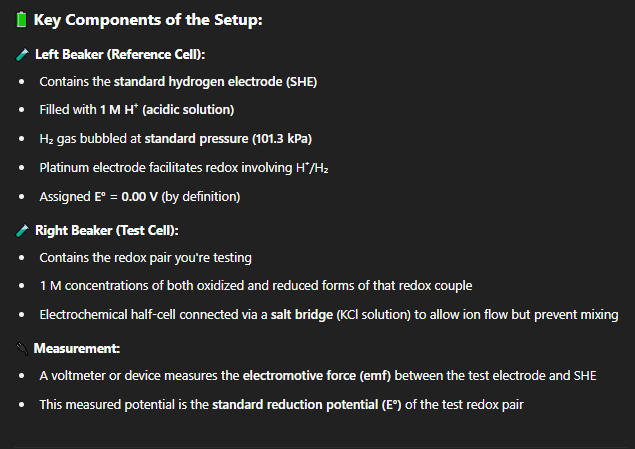
what is the term hydrogen electrode referring too?
The term hydrogen electrode is generally used for electrodes at which hydrogen gas H2 is evolved or consumed.
what is SHE mean?
explains how the standard reduction potential (E°) of a redox pair is measured, using the standard hydrogen electrode (SHE) as a reference.
SHE = universal reference point for all redox potential measurements
what is the set up for the SHE experiement?
Standard Reduction Potential (E°′):
A measure (in volts) of a molecule's tendency to gain electrons (i.e., be reduced).
More positive E°′ → strong oxidizing agent (readily accepts electrons).
More negative E°′ → strong reducing agent (readily donates electrons).
what is the strongest oxidizing agent and strongest reducing agent?
Fe³⁺ is the strongest oxidizing agent listed (most +E°′).
NAD⁺ is the strongest reducing agent (most –E°′).
formula for finding change in ∆Eo?
electron acceptor - electron donor

why is this equation important
A positive ΔE°′ gives a negative ΔG°′ → the reaction is spontaneous and can do work.
The more positive the ΔE°′, the more favorable (exergonic) the reaction.
why glucose oxidation is so powerful?

since it releases alot of energy biologically what can this energy do?
Carbon is oxidized, oxygen is reduced.
energy released by the oxidation of glucose is enormous
If all energy were released at once → it would mostly turn into heat = lethal to the cell.
How Cells Avoid This damage?
Cells need to capture (i.e. not lost as heat) as much of this energy as possible to drive other reactions. To do this the biological reactions occur in multiple enzyme- catalyzed steps (a pathway)
Mostly with small ΔG°′ (free energy changes)
Sometimes large ΔG°′ where energy is trapped
Energy is trapped as small “quanta” (e.g., ATP, NADH)
These quanta are shared across pathways to do cellular work
role of electron carriers (ECs) in oxidation reactions?
Oxidation of molecules releases lots of energy, which must be controlled.
Electron carriers (EC) allow for a stepwise release and transfer of that energy, eventually used to make ATP.
Mechanism:

what are the main water soluble electron carriers?
NAD⁺ / NADH
NADP⁺ / NADPH
FMN
FAD / FADH₂
NAD vs NADP
Both are coenzymes that participate in redox reactions.
Structurally, the only major difference is:
NAD has an –OH group.
NADP has a phosphate group (–OPO₃²⁻) in place of that –OH.
This phosphate difference allows NADP to be recognized by different enzymes and pathways compared to NAD.
Function: of both
Both NAD and NADP act as electron carriers, cycling between:
Oxidized form: NAD⁺ or NADP⁺
Reduced form: NADH or NADPH
what can they both bind to?
They bind to enzymes called dehydrogenases, which:
Catalyze oxidation-reduction reactions.
Recognize and bind NAD/NADP to a series of six alternating β-strands and α-helices on a class of enzymes called dehydrogenases
what are specific functions of NAD⁺/NADH and NADP⁺/NADPH in metabolism?
NAD usually accepts e− from catabolic degradation, & NADH delivers the e− to the mitochondrial electron transport respiratory chain.
NADPH supplies e− for synthetic pathways.
Both NAD and NADP (and their reduced counterparts) readily diffuse away from enzyme active sites
what do the oxidized forms of those electron carriers do?
Oxidized forms: NAD⁺ and NADP⁺
They accept:
2 electrons (2e⁻) and
1 proton (H⁺)
→ This bundle (2e⁻ + H⁺) is called a hydride ion (H⁻)
Site of reduction: The nicotinamide ring (pink portion in structures)
what is the half reaction for the reduction of NAD and what is it significance
A negative standard reduction potential means:
NADH is more likely to donate electrons (i.e., it’s a good electron donor).
The reaction is thermodynamically favorable in reverse, meaning NADH prefers to oxidize (release electrons).
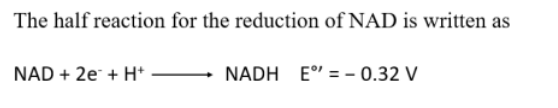
what another type of electron carriers?
FAD and FMN
whats their functions?
FAD/FMN participate in enzyme-catalyzed redox reactions.
They can accept or donate:
2 electrons (2e⁻)
and 2 protons (2H⁺)
→ This forms FADH₂ or FMNH₂ when reduced.
Key Differences from NAD/NADP:
can donate or accept two electrons and two protons
are tightly bound to their enzyme and therefore cannot diffuse away from their enzyme