MCAT Amino Acids
1/103
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
104 Terms
Glycine (Gly, G)
Non-polar, hydrophobic
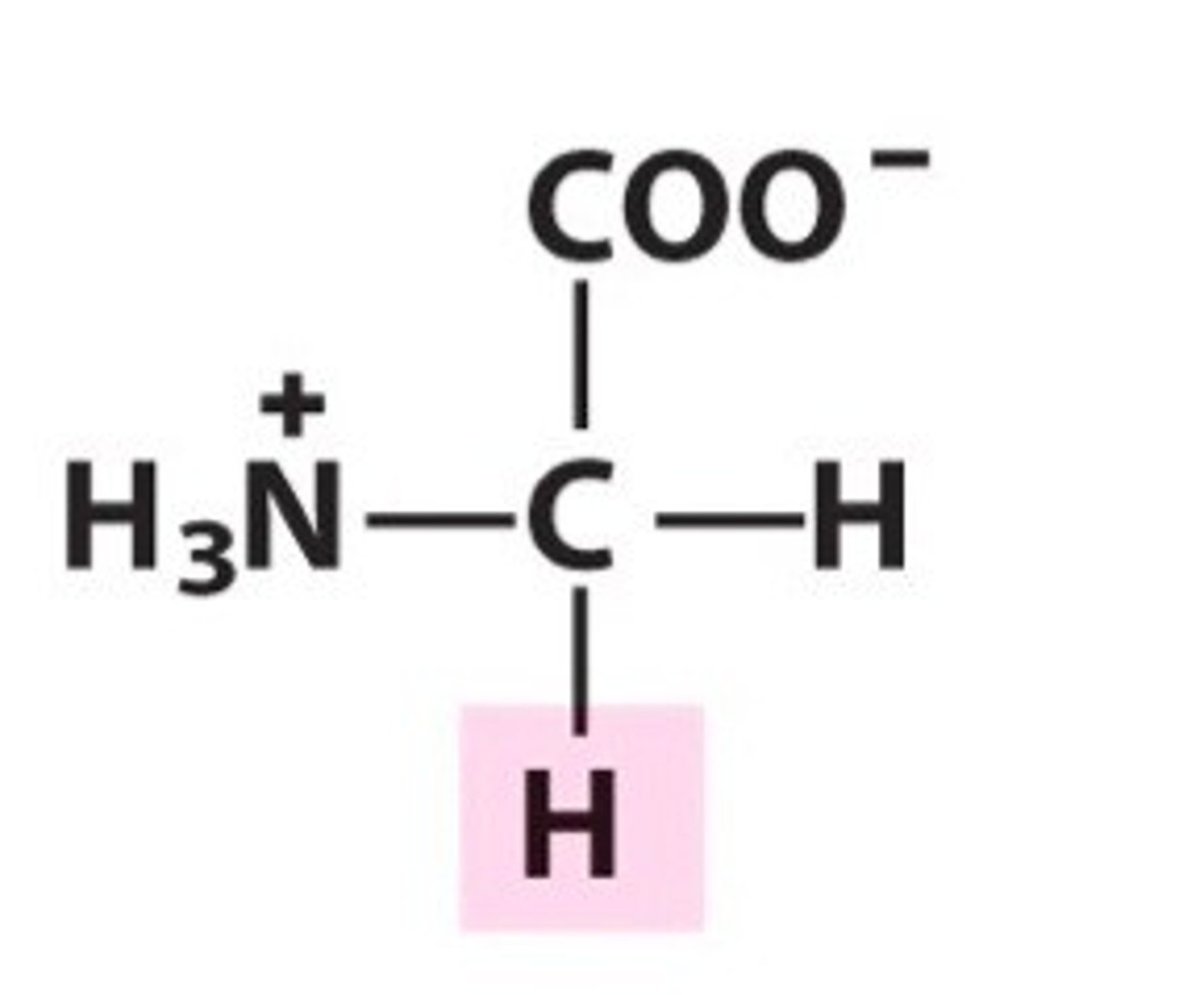
Alanine (Ala, A)
Non-polar, hydrophobic
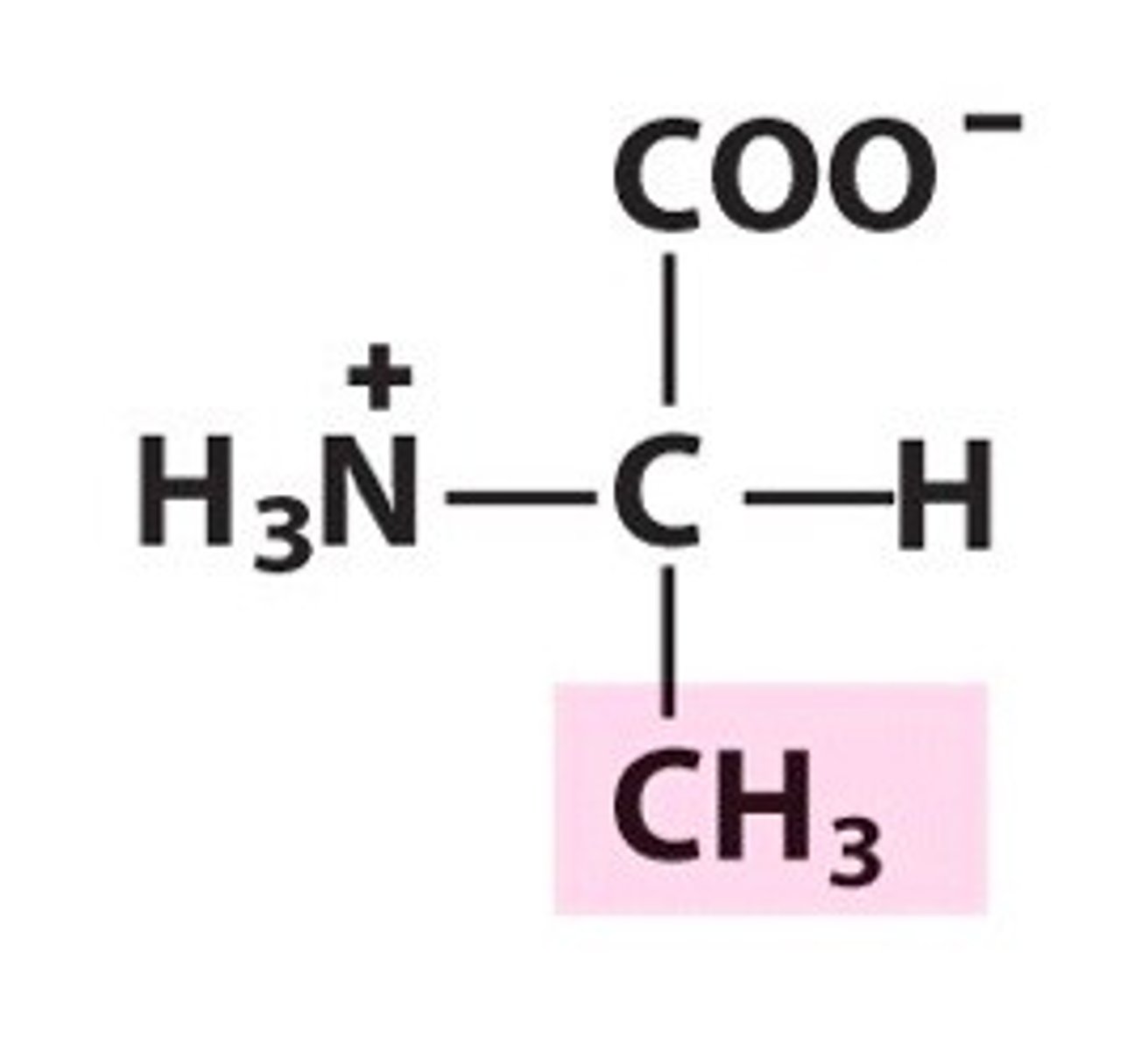
Valine (Val, V)
Non-polar, hydrophobic
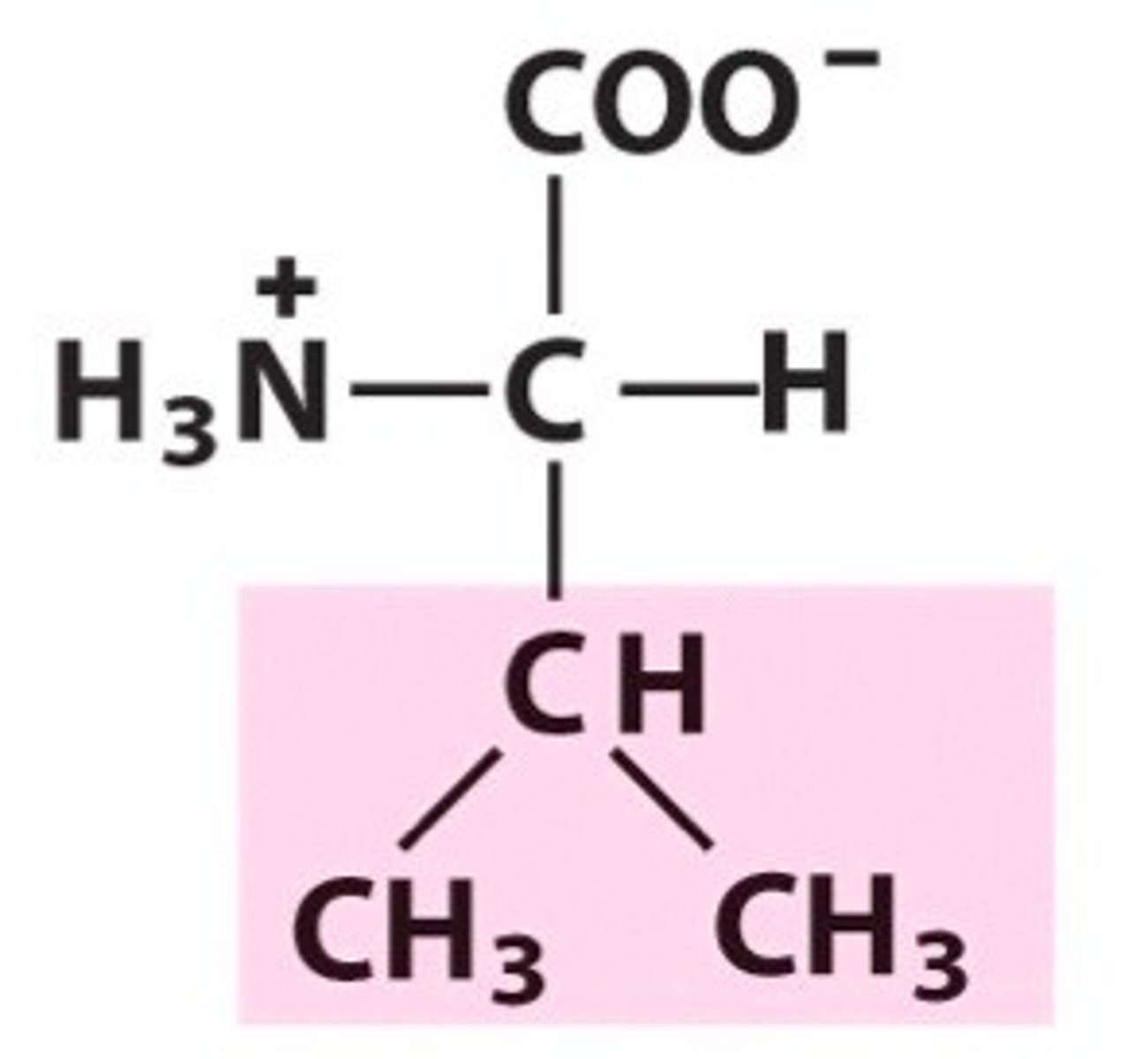
Leucine (Leu, L)
Non-polar, hydrophobic
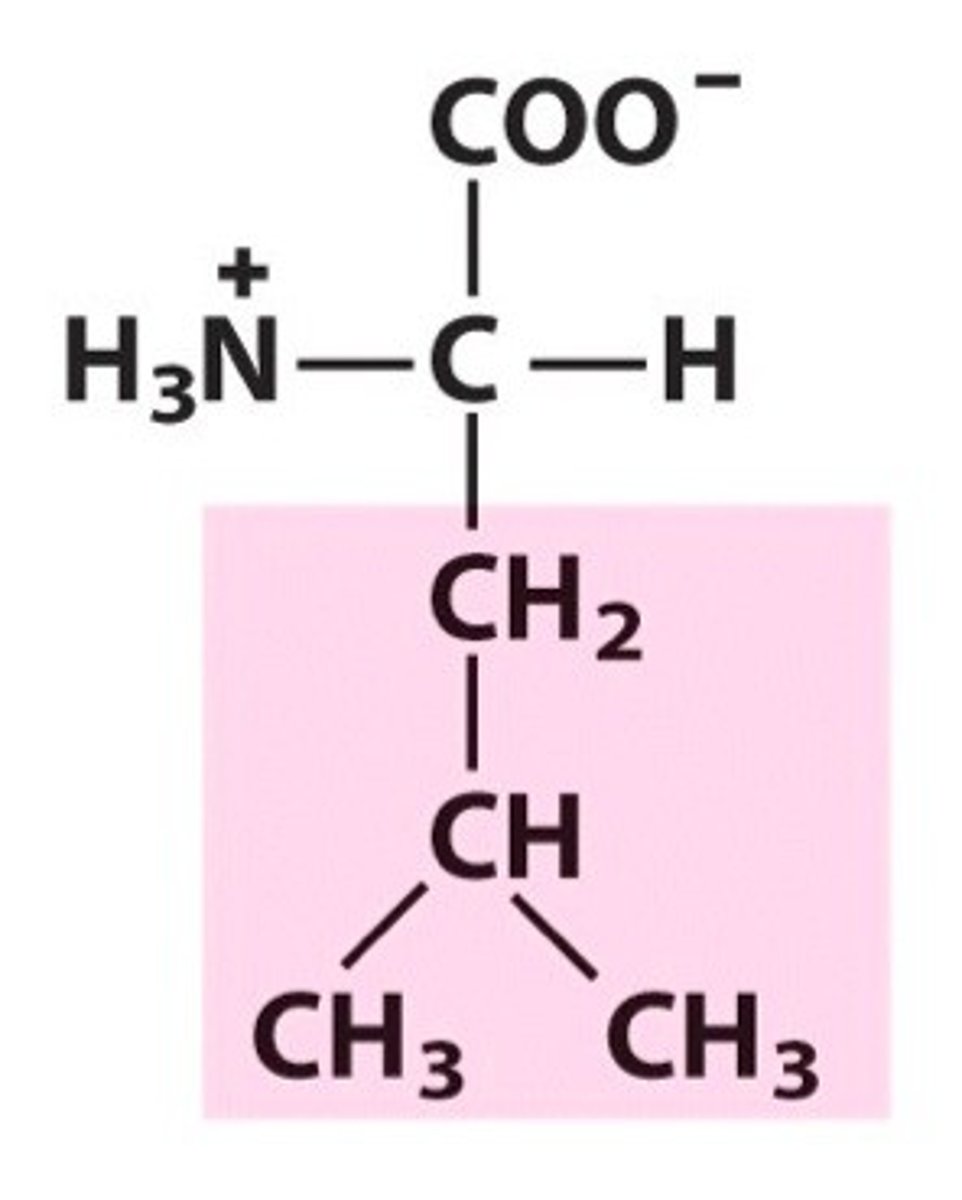
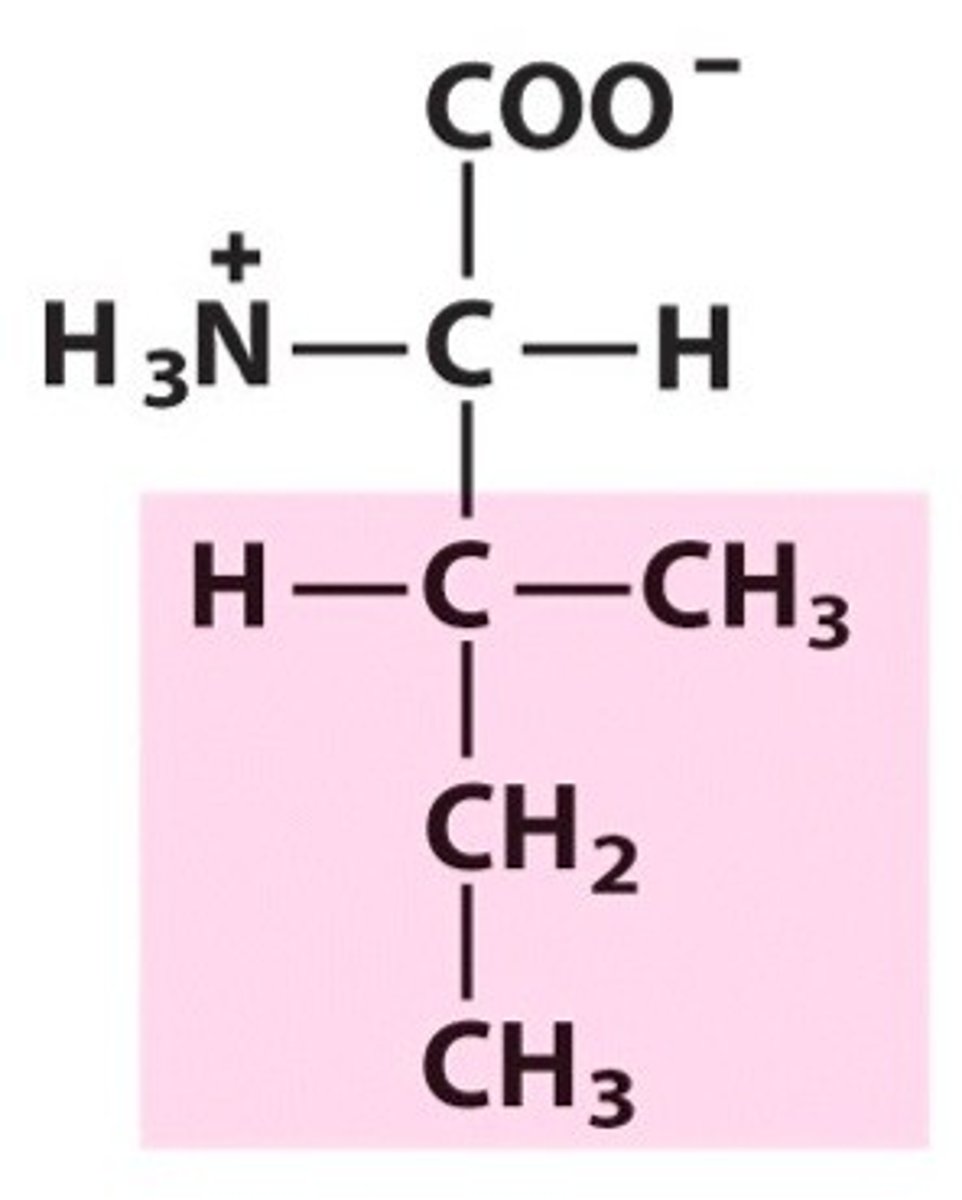
Isoleucine (Ile, I)
Non-polar, hydrophobic
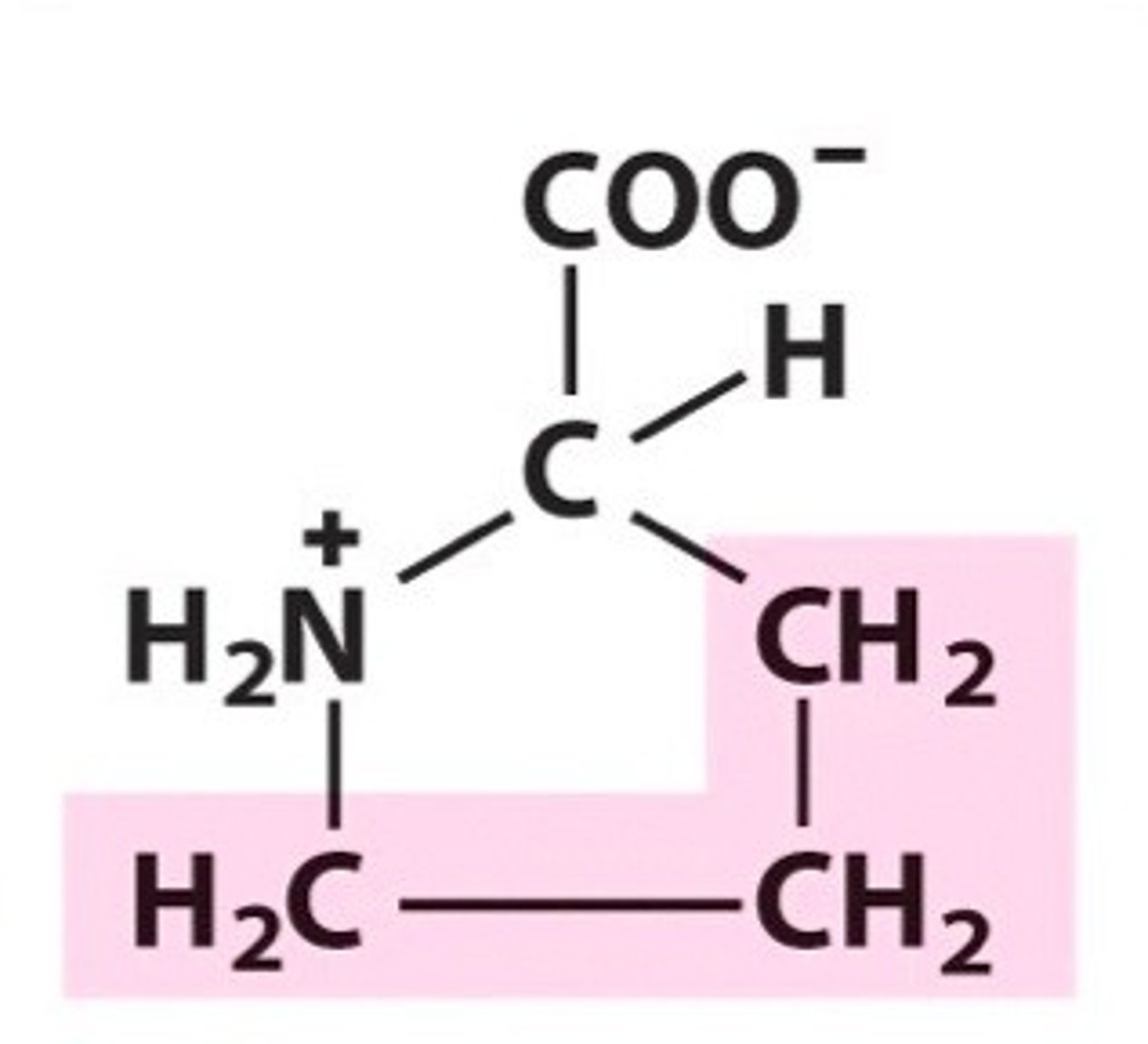
Proline (Pro, P)
Non-polar, hydrophobic
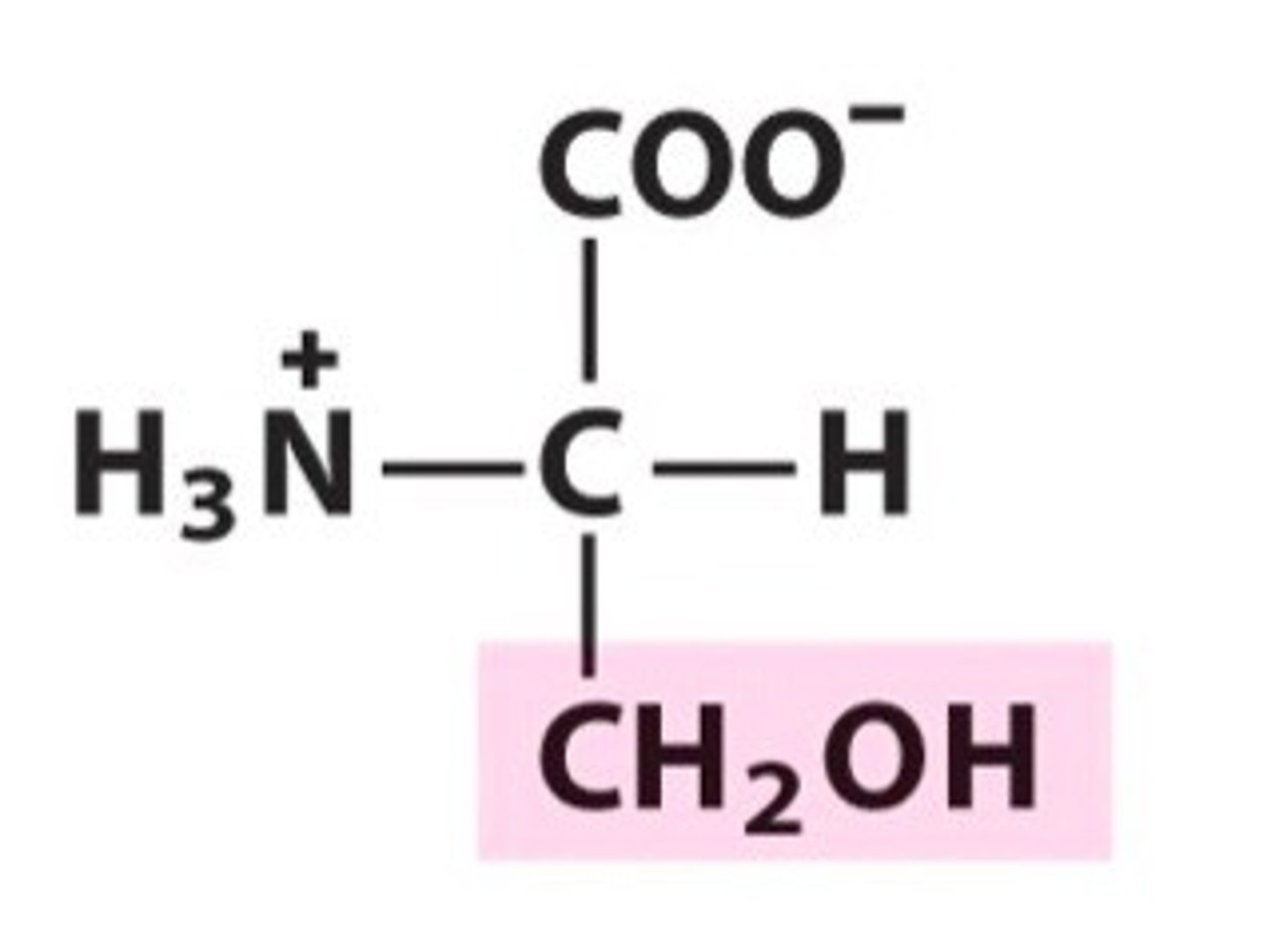
Serine (Ser, S)
Alcohol
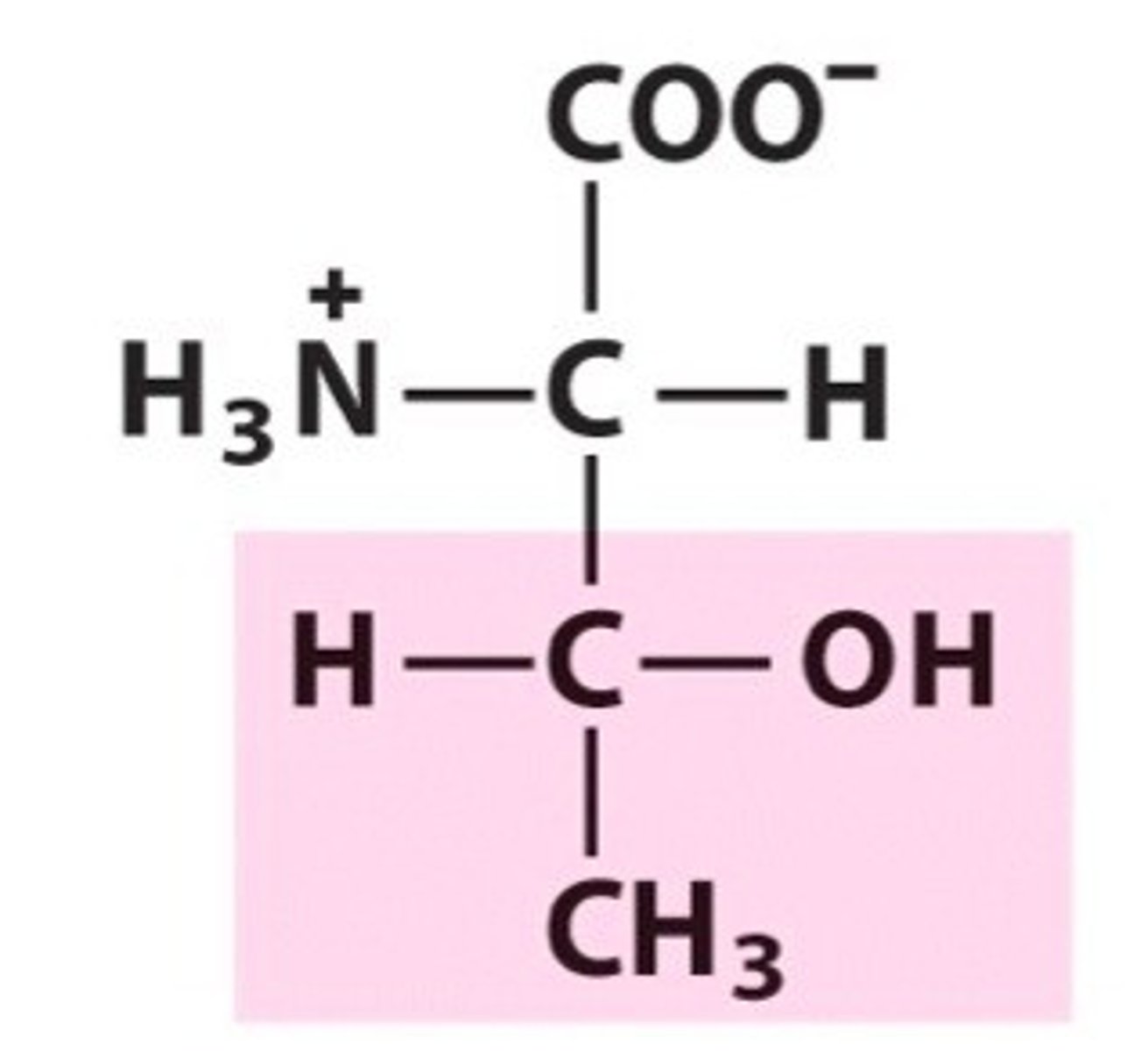
Threonine (Thr, T)
Alcohol
Phenylalanine (Phe, F)
Aromatic
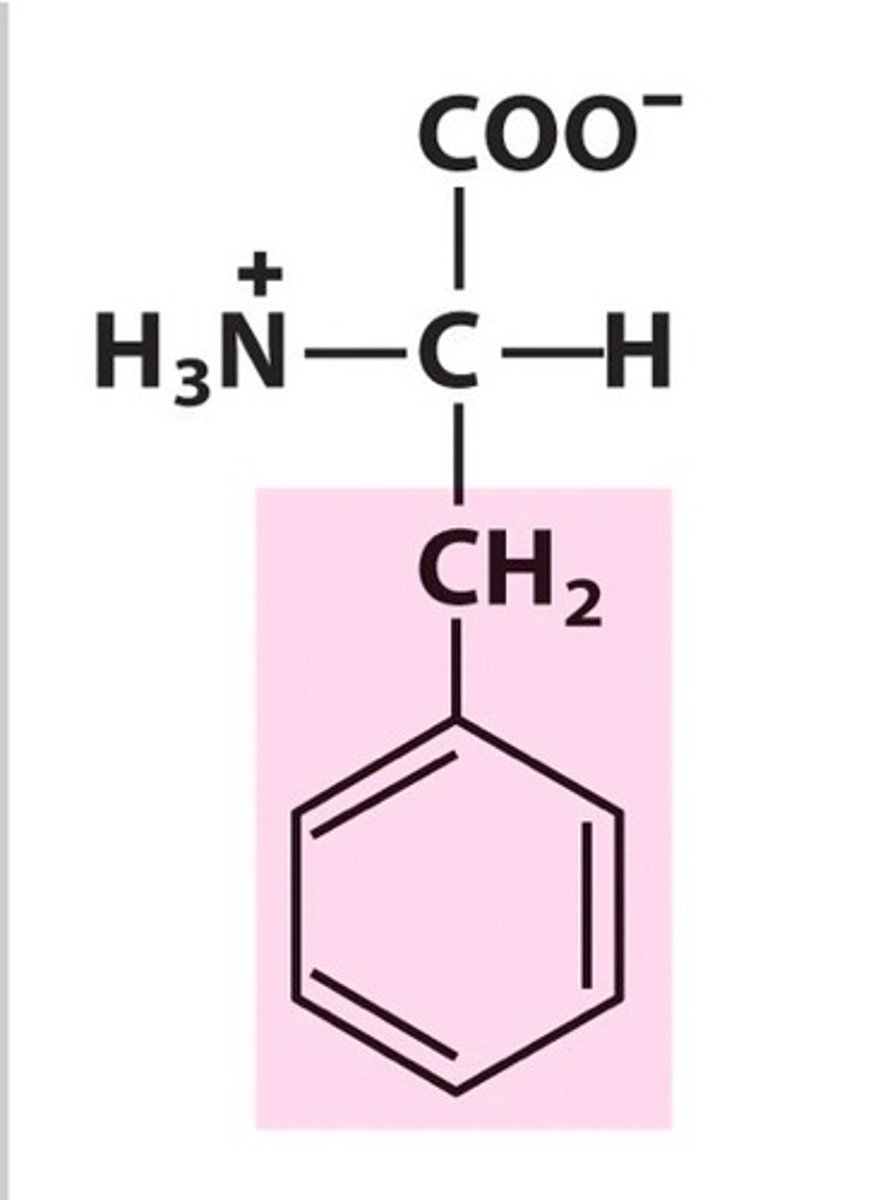
Tyrosine (Tyr, Y)
Aromatic
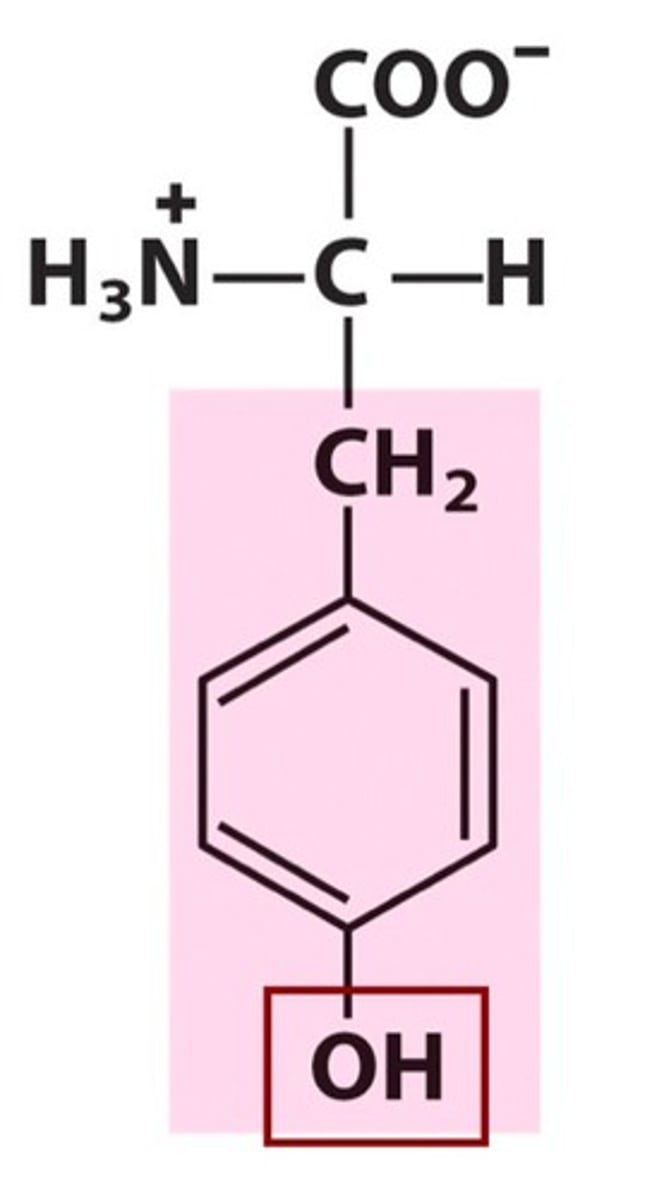
Tryptophan (Trp, W)
Aromatic
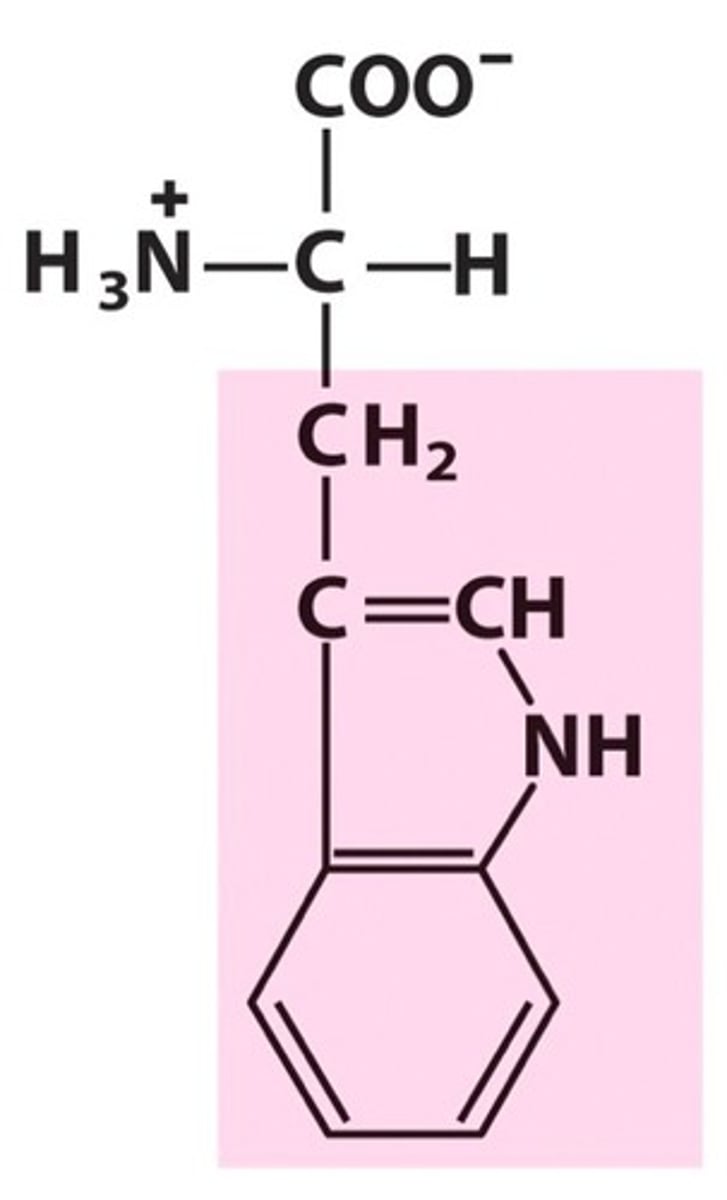
Aspartate (Asp, D)
Acid
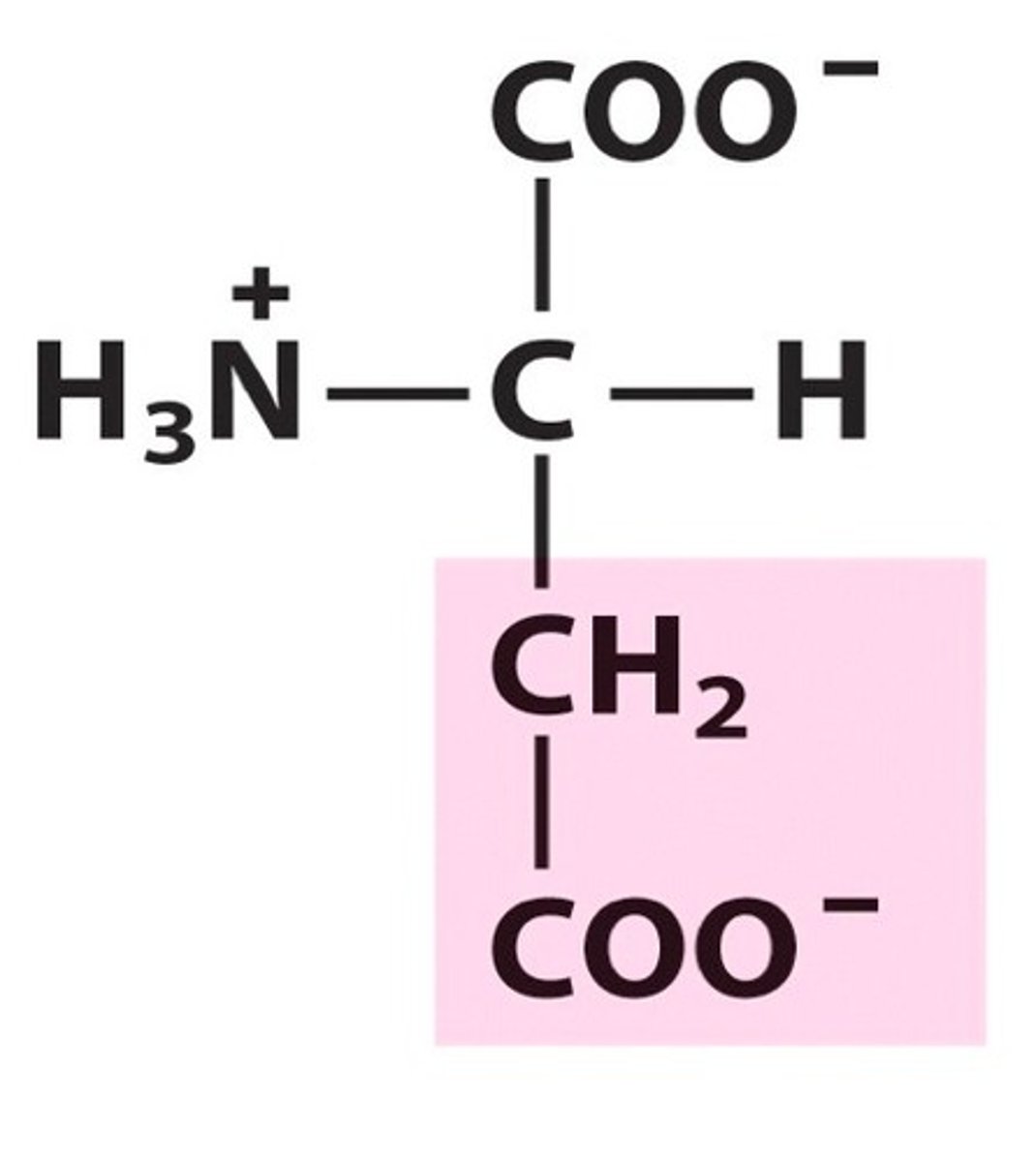
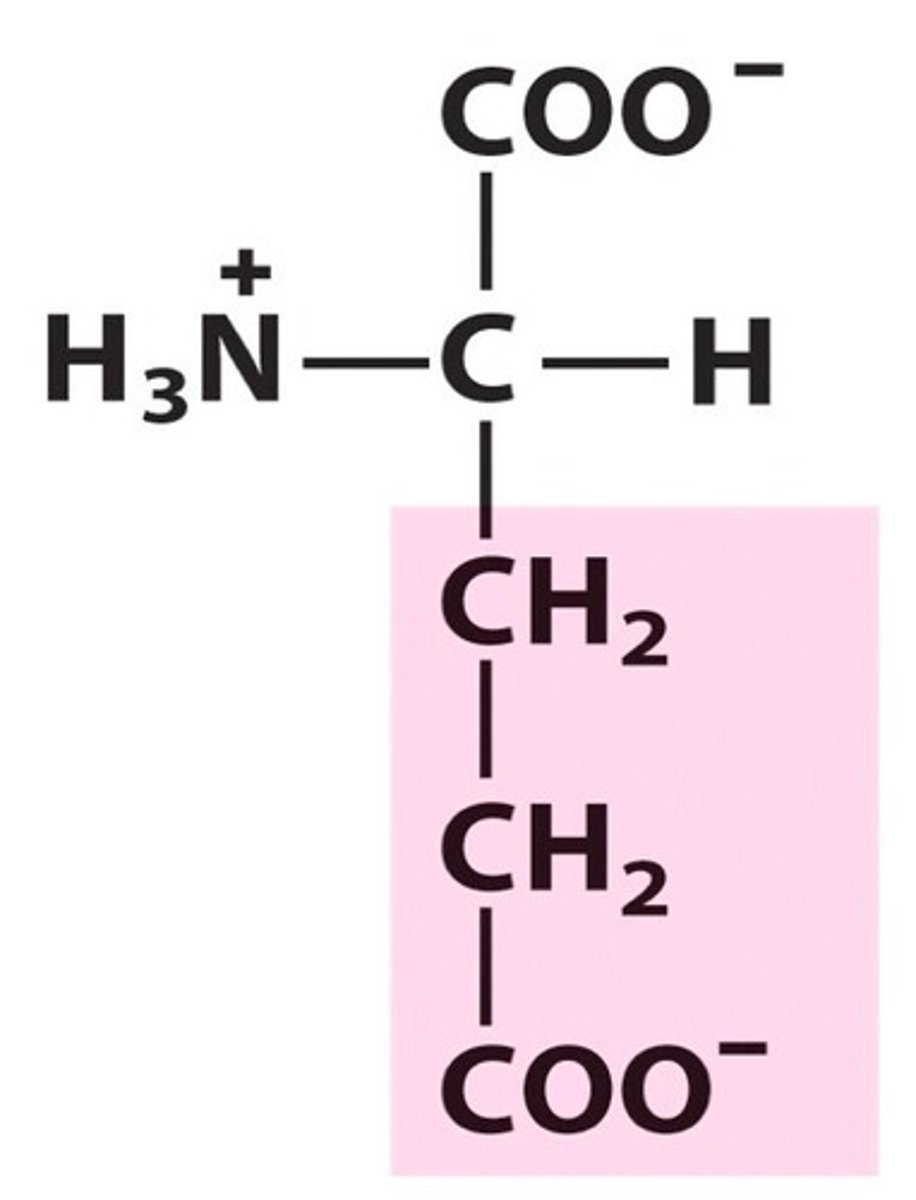
Glutamate (Glu, E)
Acid
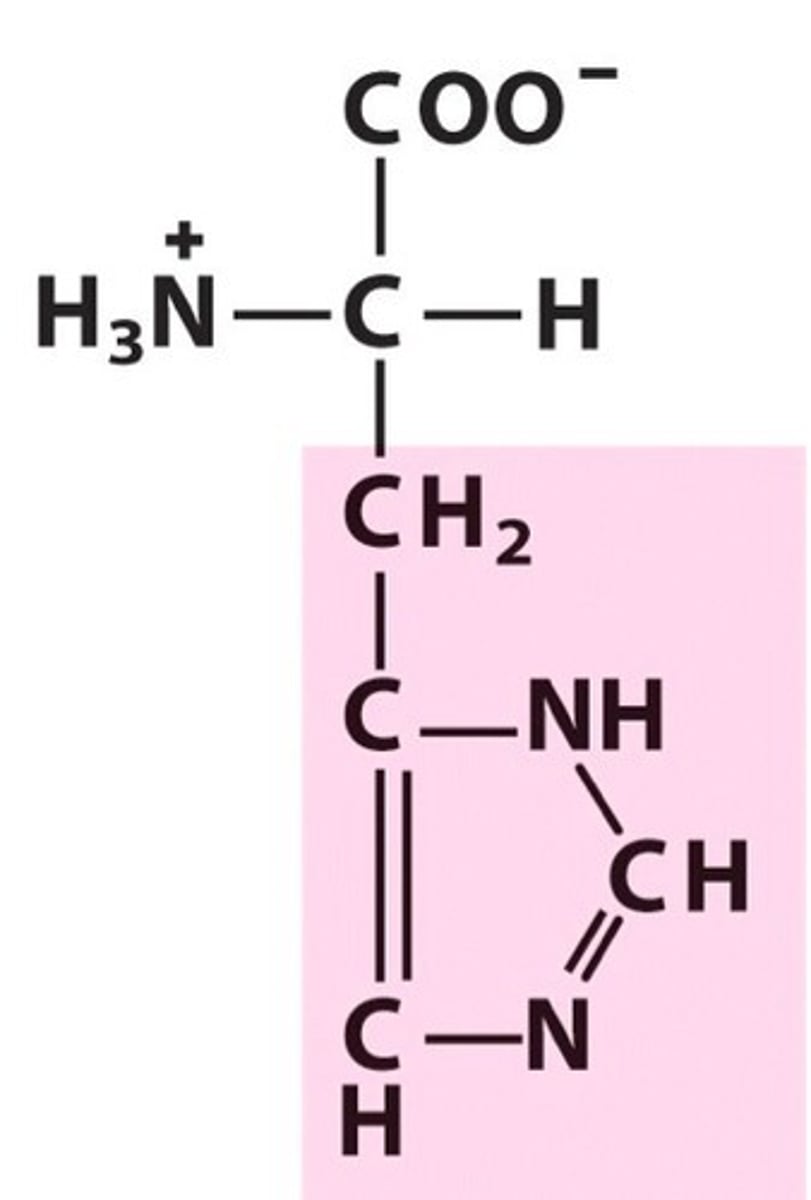
Histidine (His, H)
Base
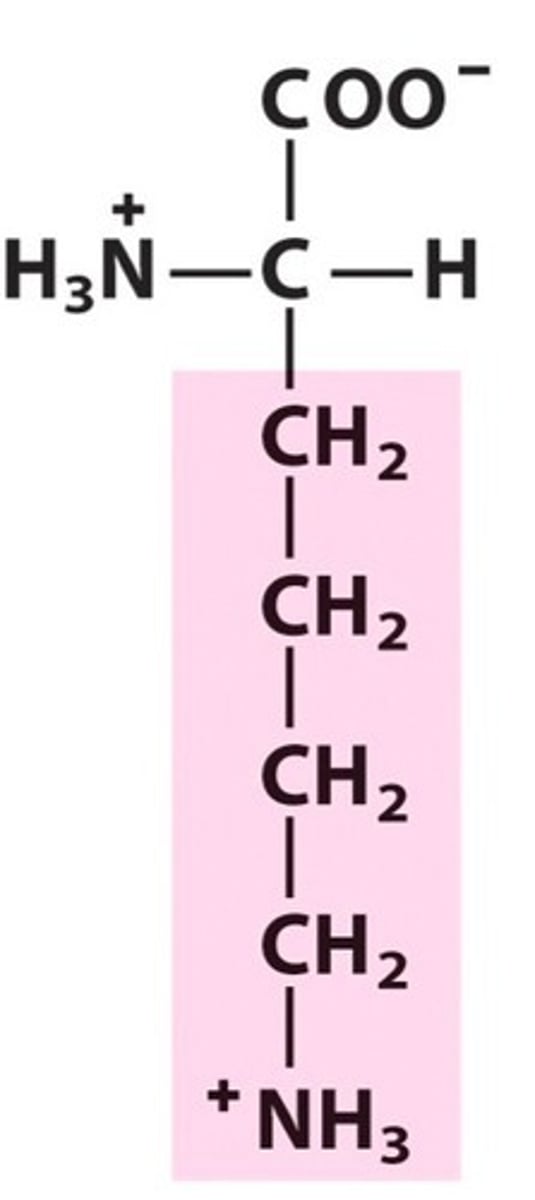
Lysine (Lys, K)
Base
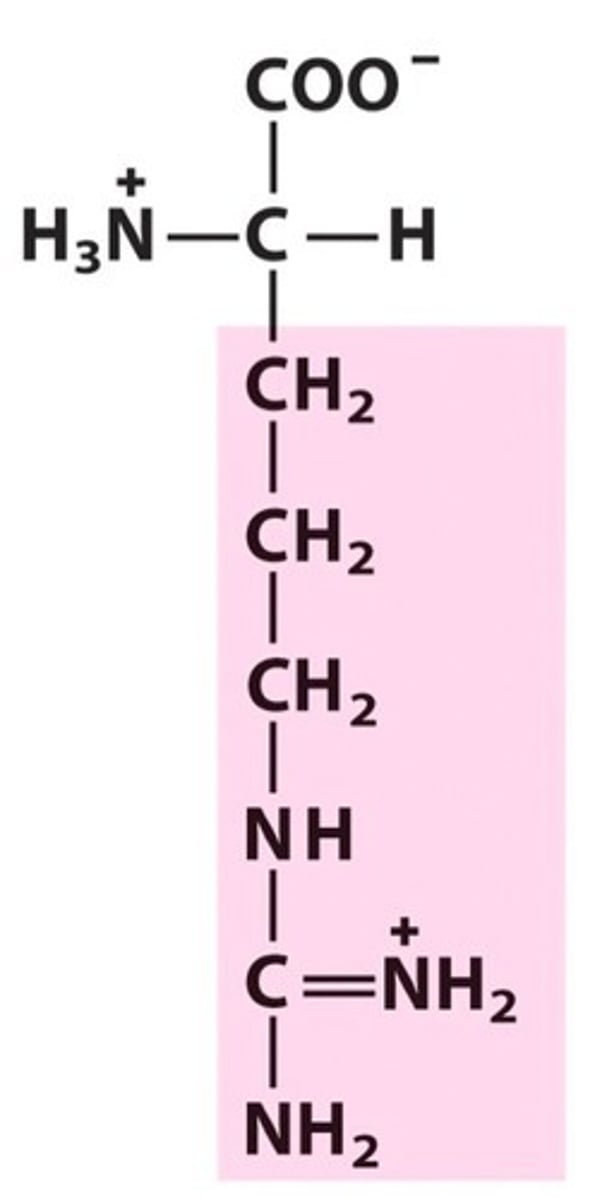
Arginine (Arg, R)
Base
Asparagine (Asn, N)
Amide
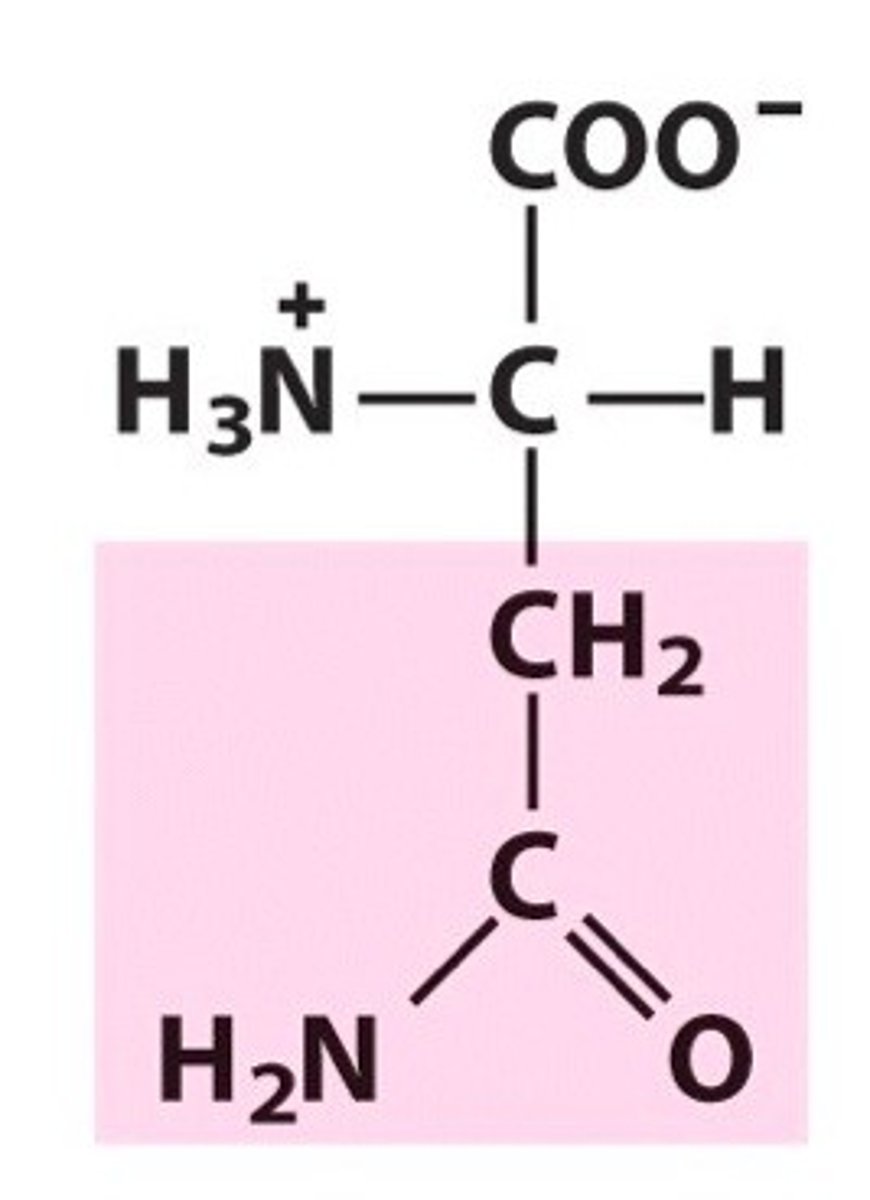
Glutamine (Gln, Q)
Amide
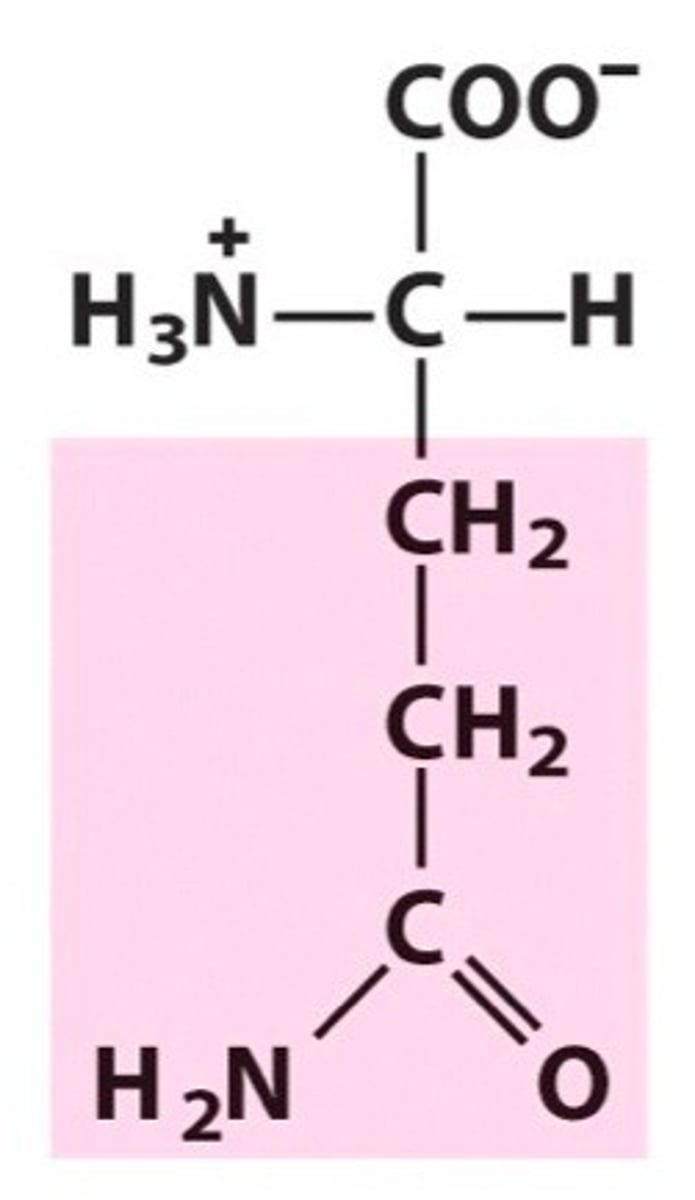
Methionine (Met, M)
Sulfur-containing
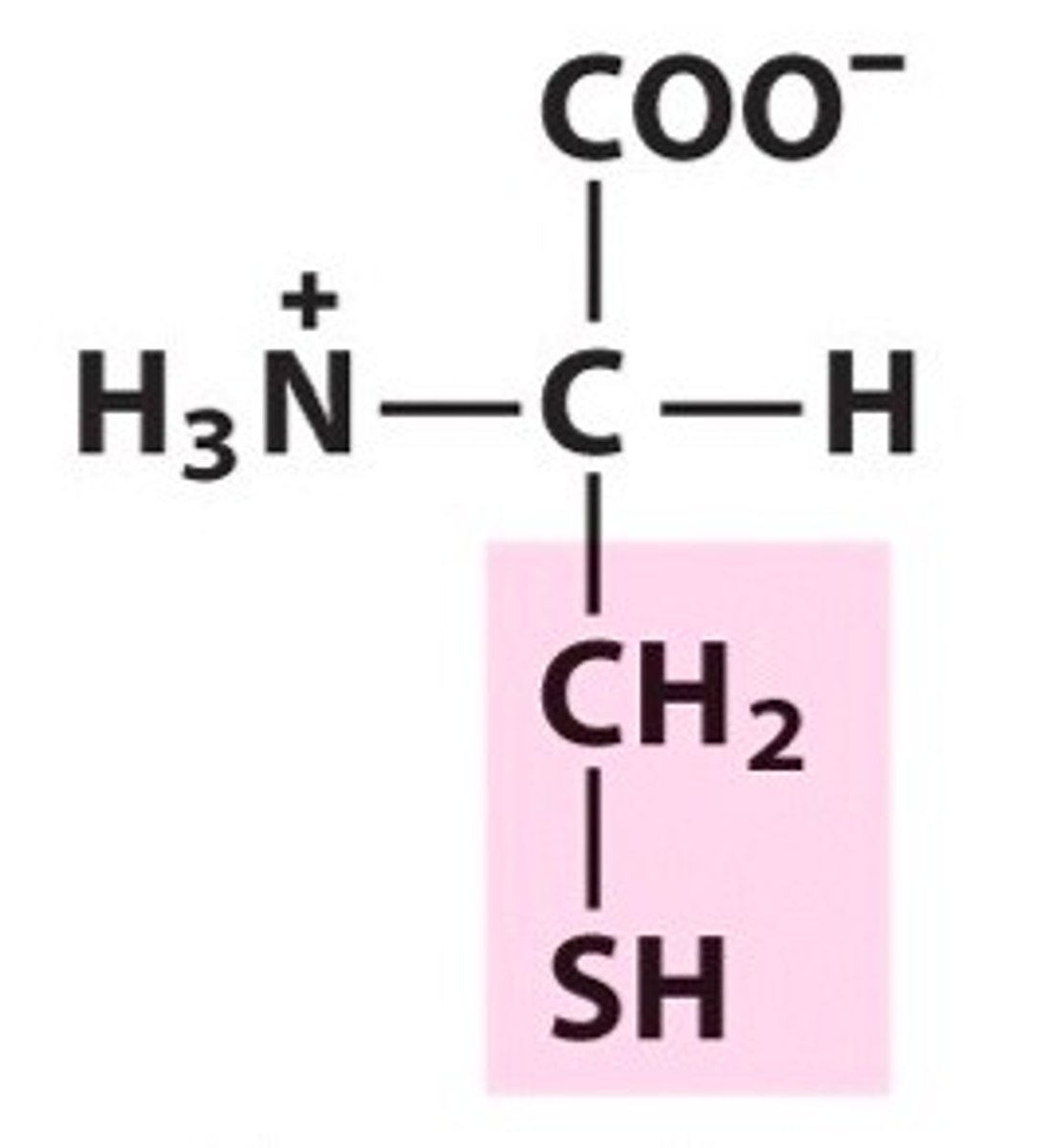
Cysteine (Cys, C)
Sulfur-containing
How many R groups are there?
19
Tho there are 20 amino acids, there are only 19 R groups, since glycine doesn't technically have one
Only achiral amino acid
Glycine
Because it is not attached to 4 different groups, glycine does not have R/S configurations or D/L
How do amino acids exist at physiological pH?
in their dipolar/zwitterion form (with a negative charge on the COO- and a positive charge on the NH3+)
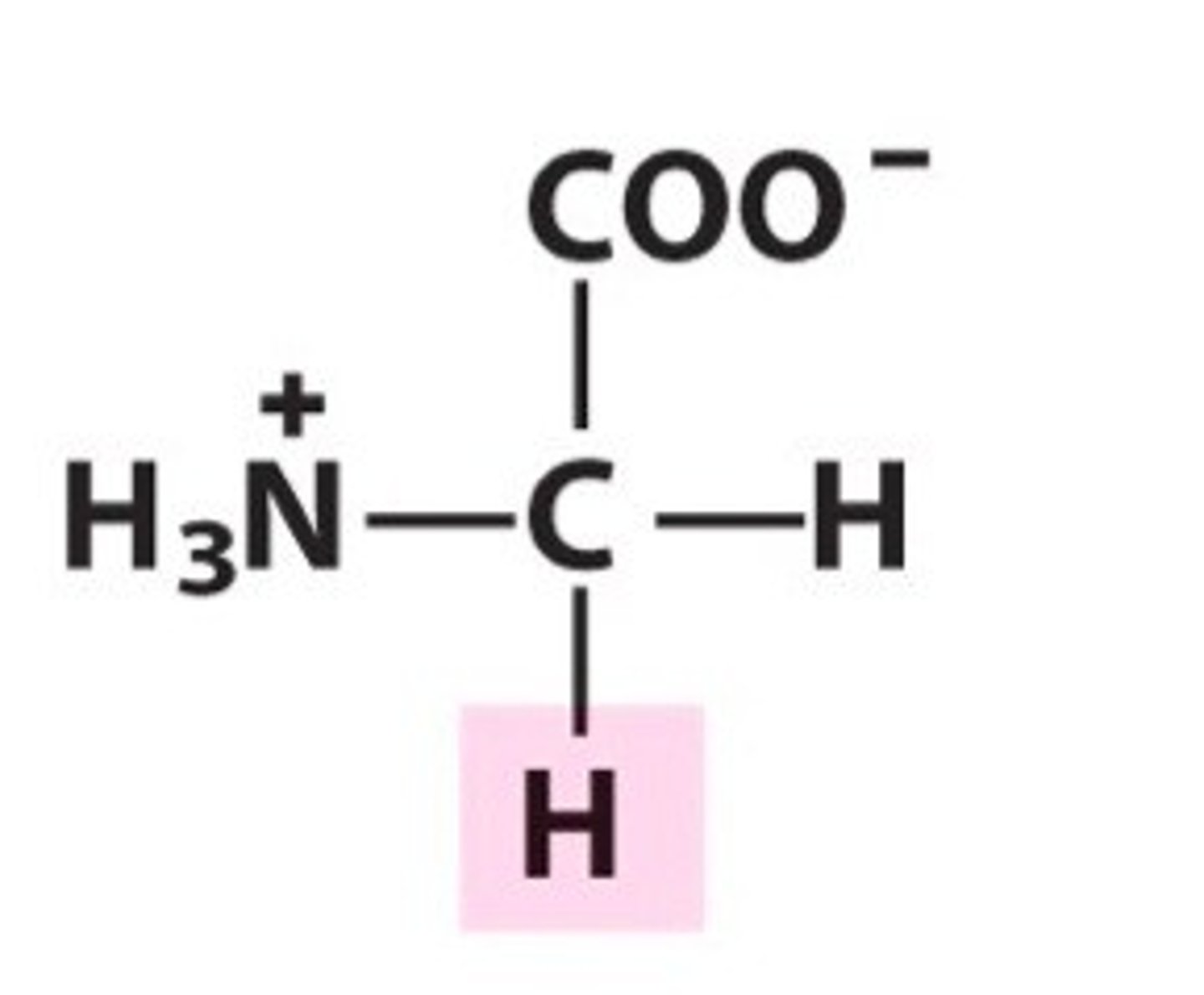
Ideal places for glycine
twists/bends, anywhere you can't fit a larger side chain
Alanine may also be found in these situations, as its methyl R group is also tiny in comparison to the other R groups.
Non-aromatic (linear) side chain
aliphatic - just Cs and Hs
Glycine (Gly, G)
Alanine (Ala, A)
Valine (Val, V)
Leucine (Leu, L)
Isoleucine (Ile, I)
Proline (Pro, P)
Link between amino acids & metabolism
Alanine (Ala, A)
can be converted into pyruvate (and pyruvate can be converted into alanine) thru transamination. If we need to create aa's from metabolic pathways or digest aa's for energy, alanine is the necessary connection - for glycolysis, gluconeogenesis & TCA cycle
Would valine be found inside an alpha helix?
NO, it's isopropyl group makes it bulky
constitutional isomers
leucine (isobutyl side chain) & isoleucine (sec butyl side chain)
molecules with same molecular formula, but differ in connection
In addition to having a chiral a carbon, has a second chiral C in the R group
isoleucine
considered an aliphatic, hydrophobic, non-polar side chain despite having a sulfur atom
Methionine -a thioether
Because the S is bound to C, the difference in polarity is not great enough to make it reactive
used as a start codon when translating from mRNA to a polypeptide
methionine
Traits of aromaticity
hydrophobic (mostly Cs and Hs interacting with no polarity to interact with)
technically has a benzyl group as its R group
phenylalanine (it is called phe bc it is a 'phenyl' group attached to 'alanine', however the methyl + benzene ring is technically a benzyl group)
has largest & most complex R group
tryptophan -- only amino acid with a bicyclo aromatic compound as its R group
It is not polar bc, tho its N technically has a partial negative charge (giving the attached H a partial + charge) its electron pair participates in resonance with the 2 aromatic structures and thus is not interested in interacting with outside groups
has an R group bound to the N in the amino side chain
proline -- important structural a.a. in polypeptides.
due to its ring, it introduces a 'kink' into polypeptide chains - forces groups toward each other and away from the ring in its R group
proline - thus it cannot be in a linear polypeptide
polar neutral amino acids -- hydrophilic due to a partial + or - charge that allows them to interact with water
Alcohols:
Serine
Threonine
Amides:
Asparagine
Glutamine
>>Cysteine & Tyrosine (even tho they're polar, are hydrophobic)
acidic amino acids
Aspartate
Glutamate
basic amino acids
Histidine
Lysine
Arginine
What makes serine polar/hydrophilic?
Its -OH group has a partial - charge on the O and a partial + charge on the H
Used for temporary, reversible binding -- bio molecules often create temporary bonds while a rxn is taking place or while sthg is being moved.
alcohols serine & threonine & tyrosine
The OH group allows H bonds to be formed rather than covalent bonds. The H bonds are weak and easily broken, or 'reversed.'
Have the ability to phosphorylate
alcohols serine, threonine & tyrosine
If a protein has to be phosphorylated, you need to create a bond to that protein/enzyme, which can happen with an OH group. The H can be discarded and the O can form a bond with the phosphate group. This is key when you're trying to turn an enzyme on or off
has chirality in its side chain
Threonine (every aa except for glycine has a chiral a carbon, but not an additional one in their R group)
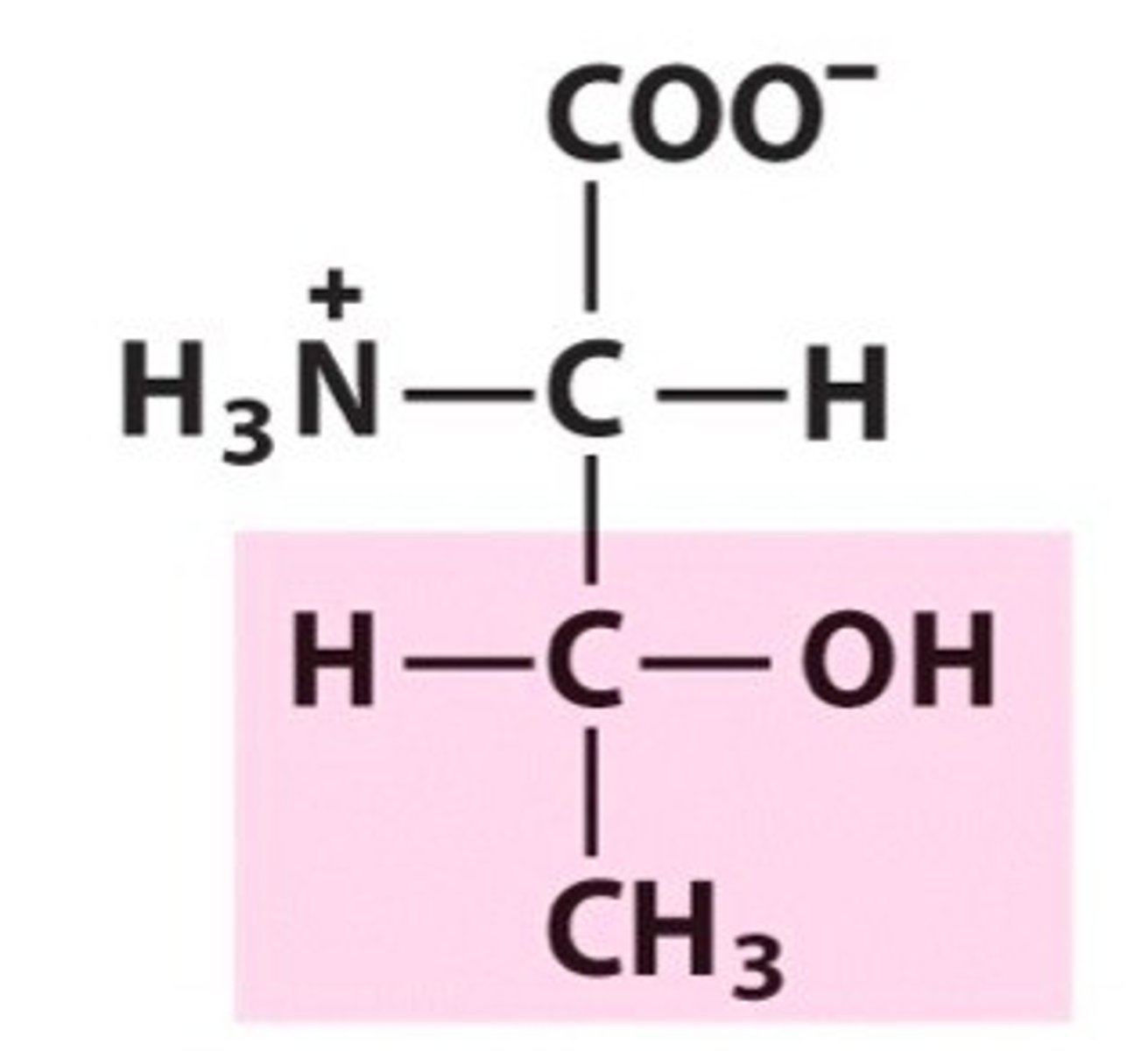
has a slight amount of polarity (due to minimal difference in negativity in its R group) but not great enough to want to interact with water
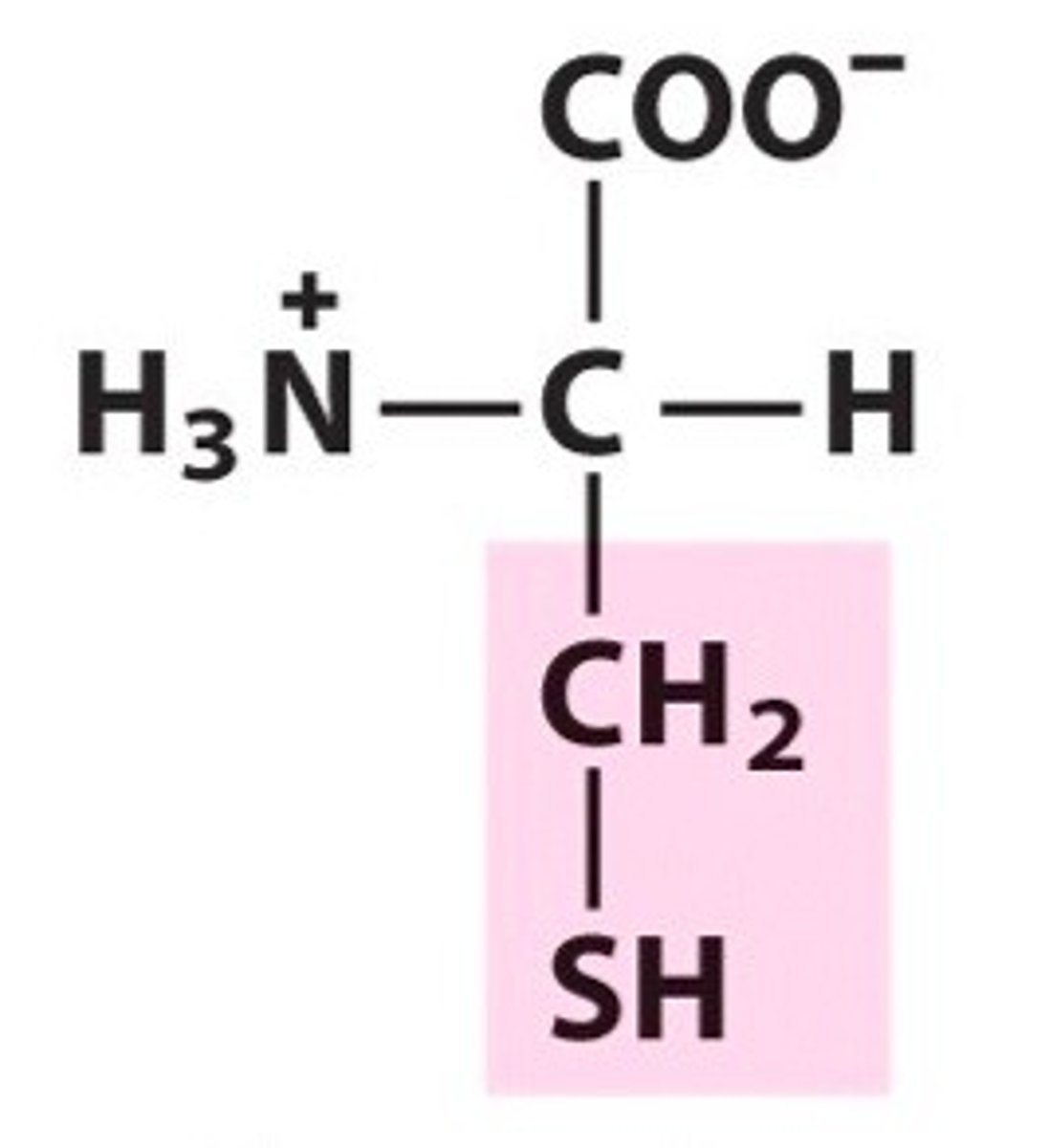
SH group in cysteine - not quite acidic or basic, but under certain conditions there is an ionizable group - the proton can be removed, giving us an S-. The S- will be hydrophobic in this case.
has the ability to form a covalent bond with another of the same aa
cysteine
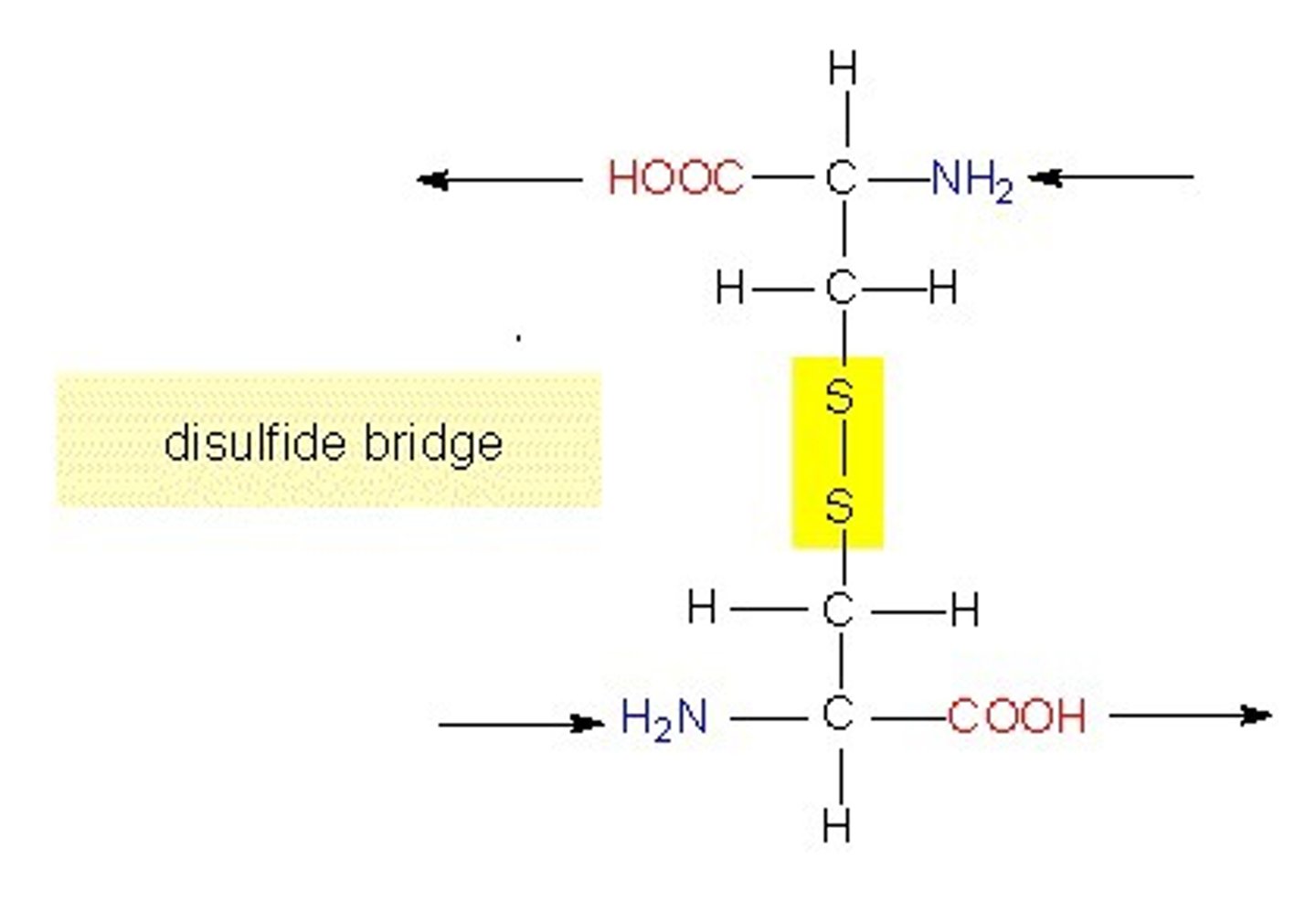
2 cysteines can oxygenate their SH groups, allowing the resulting S- to bind to each other in a covalent bond -- creating a disulfide bridge
only covalent bond seen in tertiary/quaternary structures is _. What is the purpose of this type of bond?
disulfide bridge (between 2 cysteines, or 'cystine')
helps stabilize the 3D structure of proteins/enzymes
has an OH group (polar) but the fact that it's such a tiny component of the overall R group makes the overall amino acid hydrophobic/nonpolar
tyrosine
its OH is competing with a large aromatic ring - it's not big enough to make up for the rest of the stable side chain
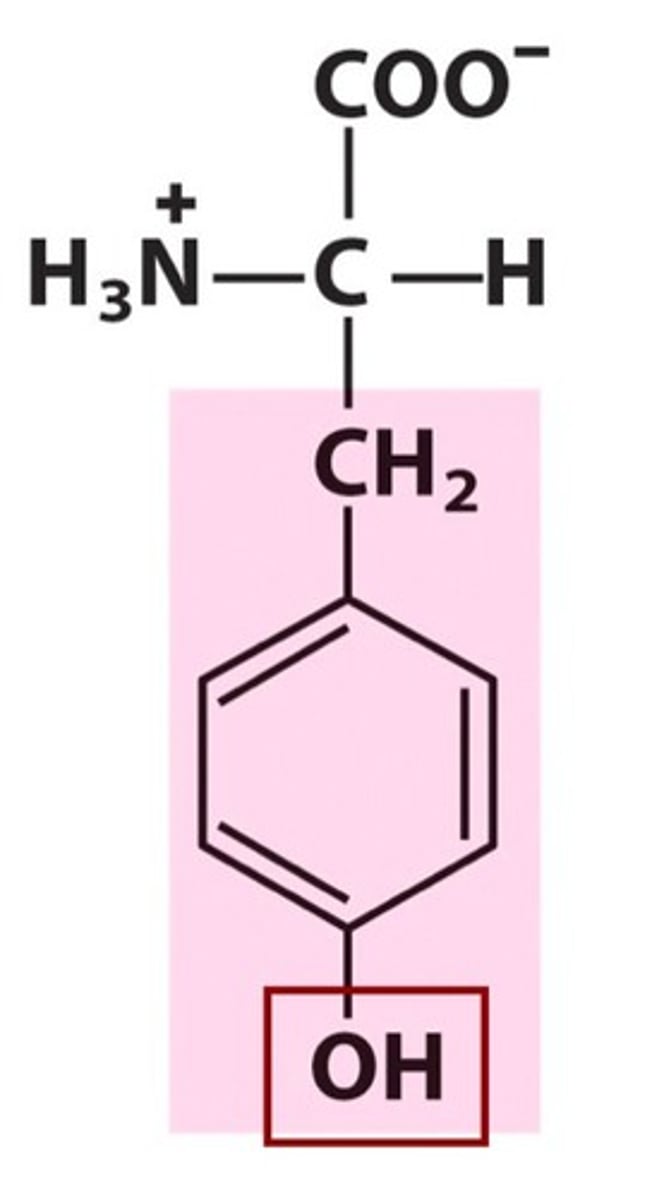
These two amino acids 'should' be basic but are not
Asparagine (Asn, N) & Glutamine (Gln, Q)
Their NH2 groups should be basic, due to lone pair of electrons on N, but sp2 Ns are far less basic than sp3 - bc electrons that would reach out & attack a proton are too busy resonating onto the carbonyl group.
play a key role in synthesis of nitrogen-containing compounds
Asparagine (Asn, N) & glutamine (Gln, Q)
The fact that they have a N in both their side chain & R group, and thus act as a N storage for living molecules. The N groups can be removed thru transamination.
If the N in asparagine is replaced by an O via transamination, we get _. If we remove the second N in the backbone, we get _
aspartate (an acidic aa)
oxaloacetate (OAA) - an intermediate in the TCA cycle
If we swap out an N for an O in glutamine via transamination, we get _. If we remove the N in the backbone, we get _
glutamate (an acidic aa)
a-ketoglutarate (intermediate in the TCA cycle)
Conjugate bases of aspartic acid & glutamic acid
aspartate & glutamate
At physiological pH, COOH groups exist as COO- - giving these aa's carboxylate groups rather than carboxyl groups.
The side chains for aspartate & glutamate look exactly like those of asparagine & glutamine, except that the amides are swapped out for carboxylic acid groups. They are intermediates in the transamination series that asparagine & glutamine participate in. What are these rxns?
Asparagine > aspartatic acid > OAA
Glutamine > glutamate acid > a-ketoglutarate
Acidic aa's (aspartate & glutamate) are typically found on the _ of proteins & enzymes bc _. They also play a key role in _ structures.
outside - they are happy to face the hydrophilic environment
quaternary & tertiary structure
Why are acidic aa's (aspartate & glutamate) key components of tertiary & quaternary structure? What are these bonds called?
They form ionic bonds between their negative side chain & a positive ion elsewhere, such as protonated basic amino acids. These ionic bonds are called 'salt bridges' and stabilize the overall 3D structure of the protein
conjugate acid of lysine's basic side chain
the NH2 is protonated to NH3+, which is how it occurs at physiological pH
Basic amino acids are found in _ bonds & _ of proteins/enzymes
salt bridges (ionic bonds, just like acidic aa's)
outside
side chain has 3 Ns, each with a lone pair of electrons
Which N is the basic one, first to get protonated?
arginine (Arg, R)
A N that can resonate is actually sp2 hybridized, thus the only N that cannot resonate is the one participating in a double bond. This means its electrons aren't distracted by resonance, making it the most basic N, the one that gets protonated. At physiological pH, there is a 2nd H added to it, giving it a + charge.
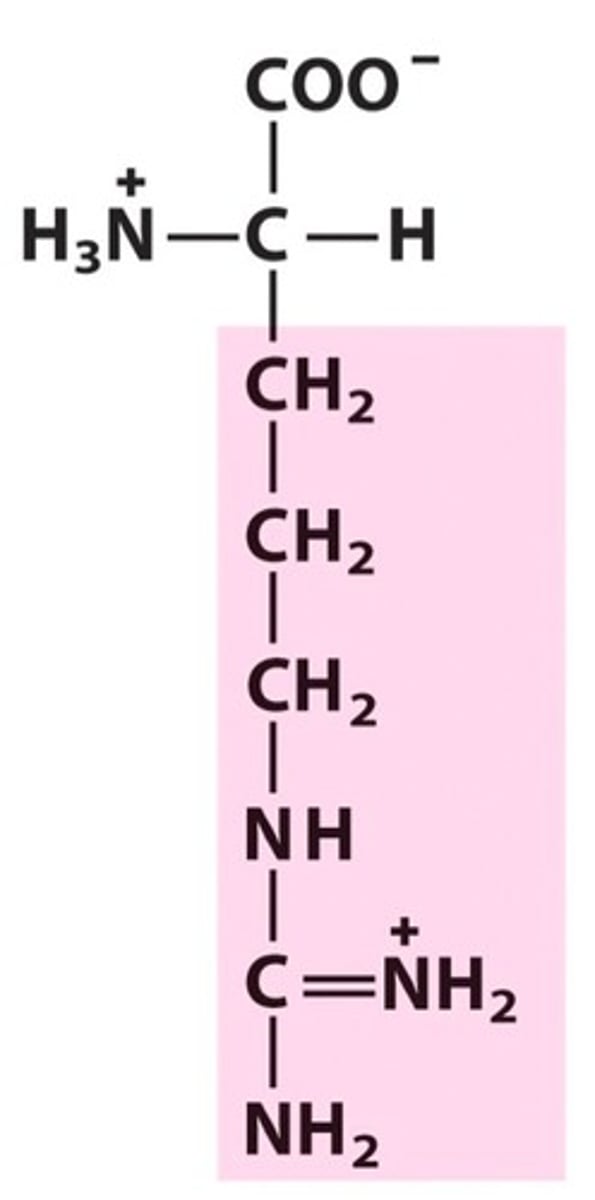
Arginine partipates in _ ionic interactions & can be found on the _ of proteins/enzymes
salt bridge
outside
Which is the basic N in histidine?
Which N is busy participating in resonance, or more importantly, aromatic resonance?
Which N doesn't need its pair of electrons, making them available to attack a proton?
If the lone pair of electrons are needed for the N to participate in resonance, it's aromatic.
The N not next to a double bond cannot participate in resonance unless it donates its electrons, making them 'busy.' The lower N already participates in resonance thru its pi bond, thus its lone pair is not needed & can be used to attack a proton.
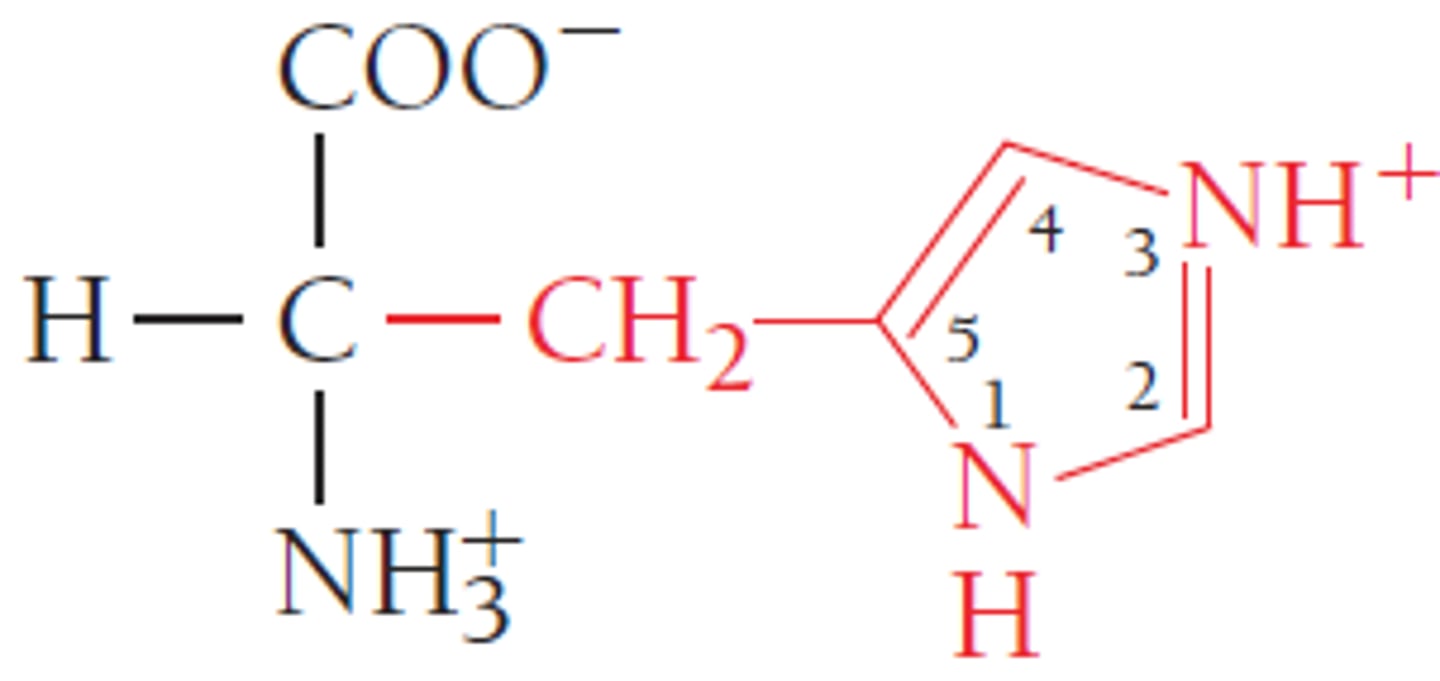
pKa of histidine side chain
~6
This is important bc it is only 1 pH unit away from physiological pH. This means that histidine is easily protonated & deprotonated in biological molecules, making it ideal in active sites of enzymes - when it's protonated or deprotonated, it will interact differently with side chains, potentially changing the conformation and therefore, the function
Chiral amino acids can be classified as _ or _ when looking at them in a 3D structure, or _ or _ when looking at them in a biological standard format
R or S
D or L
R/S do not directly relate to D/L. A D amino acid may be R or S.
A chiral carbon must be _hybridized, _ with a bond angle of _ and attached to _
sp3
tetrahedral
109.5°
4 unique substituents
To find the chirality, what do you do?
Rank substituents from high to low priority
Put the lowest priority in the back (bc we're only looking at the 3 highest)
Determine whether you go CW or CCW to go from 1 to 2 to 3
In ranking substituents of amino acids, what is the order of the 5 most common atoms seen?
S > O > N > C > H
If faced with 2 of the same, cross that out & go to the next atom.
R and S are _ of each other
What is the 'swap' method?
enantiomers
when you swap 1 group to get the enantiomer (if you swap the position of the #3 and #4 substituents so that #3 is in the 'away' position, you will get the other enantiomer)
How to find whether an amino acid is D or L
Rather than looking at the OH group, we put the COOH group at the top (just like sugars) and look at the NH2 group to determine D or L.
If NH2 is to the right, it is D
If NH2 is to the left, it is L
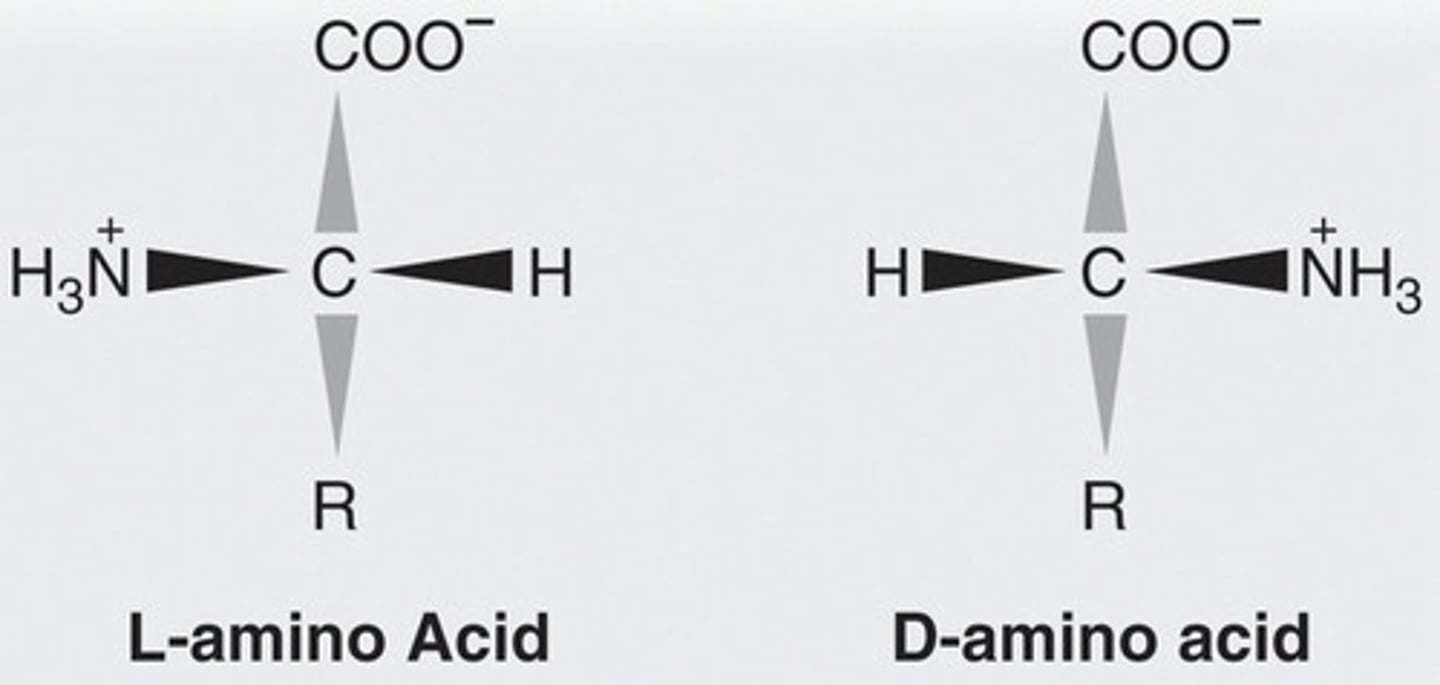
Biological amino acids prefer which form: D or L?
L
The neutral form of amino acids cannot exist in nature bc of _
the acid-base interactions
The acidic COOH will deprotonate to COO- and the basic NH2 will be protonated to NH3+. In nature, they won't interact with each other, but rather with the solution.
This a Zwitterion, or dipolar ion (2 charged groups on molecule). Acidic/basic side chains can have a 3rd charge in their R group
donate H+
acids
accept H+
bases
How to find charge of an aa at any given pH
Look at it in its neutral form (NH2, COOH) and look for the pKa values - these will tell you at which pH it's going to gain or lose a proton
What is the pH of any ionizable group and will it be protonated or deprotonated based on the pKa?
relationship between pH & pKa
pKa = -logKa
pH = pKa + log[conjugate base]/[acid] <
Ka =
[H+][A-] / [HA]
**Ka is proportional to the H+ concentration, which tells us the pH
What does pKa tell us?
the likelihood of a molecule to take or donate a proton from/to solution
What does pH tell us?
concentration of H+
low [H+] = acidic = lots of H+ to be pumped onto molecules dissolved in solution
High [H+] = basic = lots of OH- to take protons away from molecules dissolved in solution
We have to compare the acidity or basicity of the solution to the molecule's desire to hold onto their H+?
When pH < pKa, what does that tell us?
acidic solution
The molecule is stronger and takes the proton away from solution - and will be protonated
When pH > pKa, what does that tell us?
basic solution
The solution is stronger and takes the proton from the molecule, deprotonating it
When pH ~ pKa, what does that tell us?
'stale-mate': buffer zone
The solution wants the proton just as much as the molecule.
If pH = pKa, what does that tell us?
buffer zone >> 50:50 - half molecules will be protonated and half will be deprotonated.
If pH is within 1 unit of pKa, what does that tell us?
still in the buffer zone, but the molecules will be slightly more or less protonated depending on direction (if pH is lower, you'll have more protonated; if pH is higher, you'll have more deprotonated - but still in that partially charged region)
What does the pKa of acetic acid (4.8) tell us?
Acetic acid is happy to donate its proton depending on the solution. If dissolved in an acidic solution, we have too many protons & Le Chatelier's principle says the rxn shifts to the left & we protonate acetic acid.
If dissolved in basic solution, the base (OH-) is pulling protons out of equilibrium and the rxn shifts to the right - we deprotonate the acid
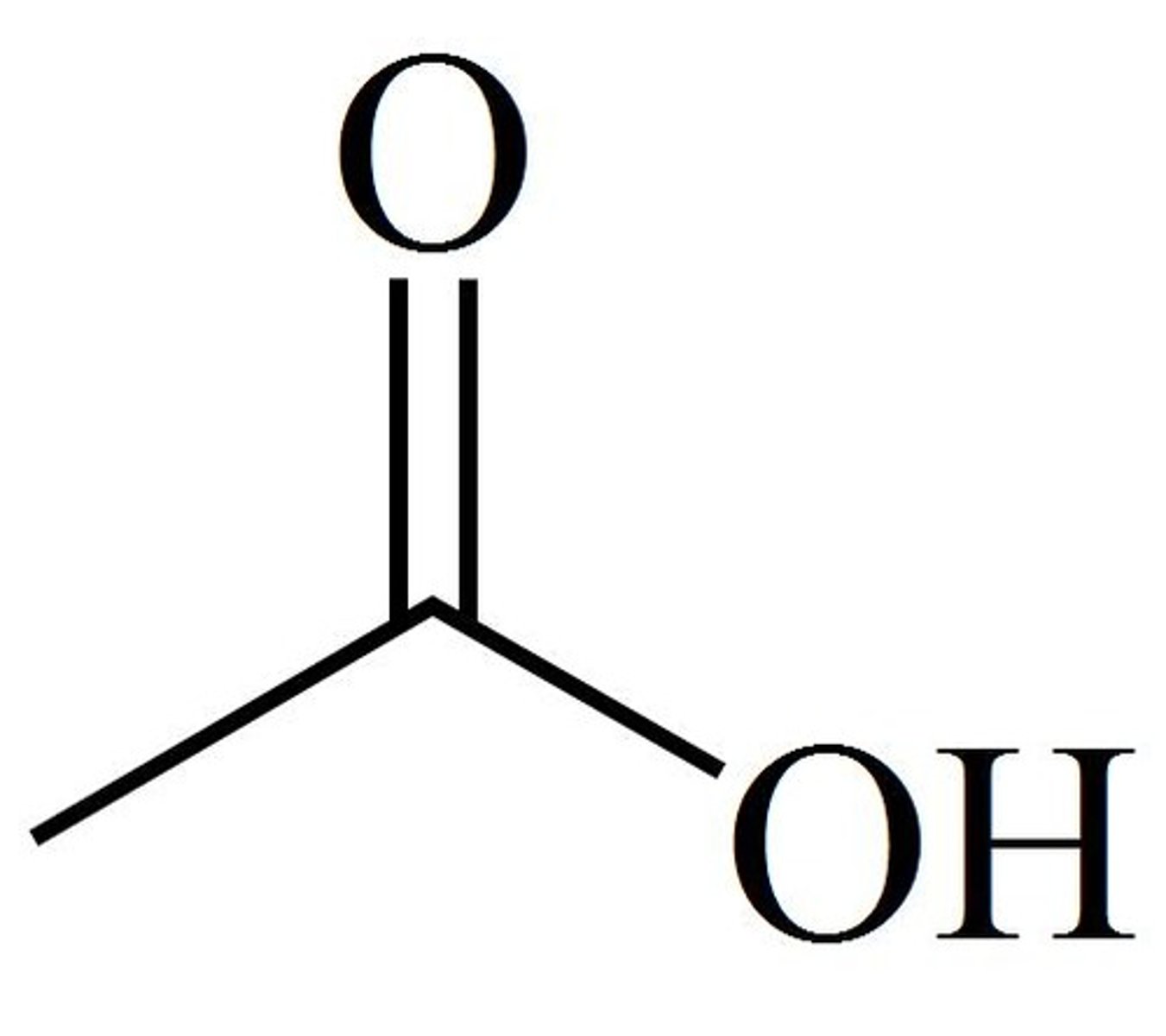
If acetic acid (pKa = 4.8) is dissolved in solution with pH 2, what happens?
pH is significantly lower than pKa. We have so many protons in solution that we will protonate every available acetate to get acetic acid. The charge = 0, a neutral molecule.
If acetic acid (pKa = 4.8) is dissolved in solution with pH 4.8, what happens?
buffer: 50% protonated, 50% deprotonated
The charge in this case (average charge) equals -0.5. We have a charge of 0 for the protonated form and a charge of -1 for the protonated form, thus the avg charge is -0.5.
As pH goes up, charge becomes _. As pH goes down, charge becomes_ with the ideal buffer range within _ the pKa value.
more negative
less negative
1 pH unit above or below
If acetic acid (pKa = 4.8) is dissolved in solution with pH 3.8, what happens?
There is a 10:1 ratio of protonated to deprotonated
If acetic acid (pKa = 4.8) is dissolved in solution with pH 8, what happens?
We have a very basic solution, with OH- more desperate for the proton than the acetic acid. OH- will take away all the protons, and we have acetate in solution for a charge of -1
The pKa of the carboxy side chain will vary between amino acids, depending on what else is nearby, thus the specific charges will vary
If methyl ammonium ( CH3NH3+, pKa = 10.6) is dissolved in solution with pH 7, what happens?
The pH is significantly lower than pKa and is much more acidic (it has so many H+ that it doesn't want any more). Thus, the methyl ammonium is more hungry for the proton and will be primarily protonated (CH3NH3+) - charge is +1
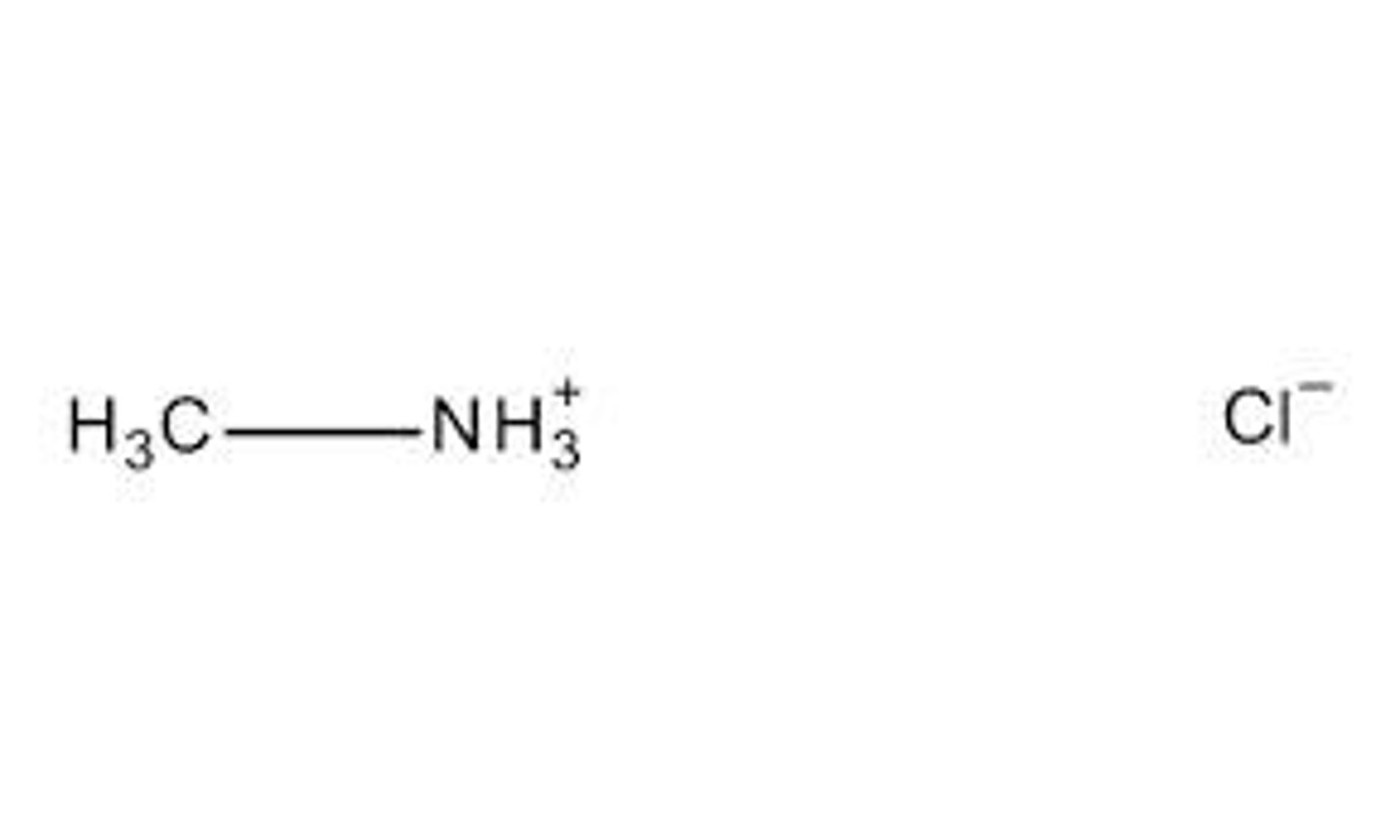
If methyl ammonium (CH3NH3+, pKa = 10.6) is dissolved in solution with pH 13, what happens?
The pH is significantly greater than the pKa, thus methyl ammonium will exist primarily in deprotonated form (CH3NH2), with a neutral charge. It's not neutral bc we started out with a positive charge; dropping one unit brings us to 0, or neutral.
If methyl ammonium (CH3NH3+, pKa = 10.6) is dissolved in solution with pH 10, what happens?
pH is slightly less than pKa, making it slightly more acidic. However, we're still in the buffer zone (pH within 1 unit of pKa). We will have slightly more CH3NH3+ than CH3NH2, with a charge somewhere between 0 and +1.
If glycine (COO- pKa = 2.3, NH2 pKa = 9.6) is in solution of pH1, what happens?
pH 1 is less than both pKa values. Thus the solution has more protons, and will give them to both the amine and carboxyl groups. Thus the groups exist as NH3+ and COOH. If we add both charges, we get +1 and 0, for a net charge of +1.
If glycine (COO- pKa = 2.3, NH2 pKa = 9.6) is in solution of pH 2.3, what happens?
The carboxyl group will be partially protonated/partially deprotonated (half will have a -1 charge and half will be neutral, for an avg charge of -0.5). The amine pKa is greater, thus all will exist as NH3+ with a +1 charge.
If glycine (COO- pKa = 2.3, NH2 pKa = 9.6) is in solution of pH 7, what happens?
We expect to see the Zwitter ion at physiological pH.
This pH is greater than the carboxyl pKa, thus it will exist as COO- with a -1 charge. The pH is less than the amine pKa, thus it will exist as NH3+ with a charge of +1. This gives a net neutral charge for the molecule
As we raise pH, we went from +1 and we're dropping half a point. If we raise
What is the isoelectric point (pI)?
iso = same
electric = charge
It is the pH at which the amino acid has a net charge of 0. For ex, amino acids such as glycine exist at a net charge of 0 at physiological pH, as the - charge on the COO and the + charge on the NH3 cancel each other out
pKa for carboxyl group of amino acids
2
pKa for amine group of amino acids
10
If an amino group doesn't have a pKa for its side chain (such as glycine), how do you find pI?
pI = (pKa1 + pKa2) / 2
Take the avg of the pKa values
pI(glycine) = (2.3 + 9.6)/2 round > (2+10)/2 = 6
If an amino group has 3 pKa values (1 for its side chain in addition to backbone groups), how do you find pI?
guess-&-check method: look at the pH and determine what charge each group will have
Ex: tyrosine (pKa COO: 2.3, pKa NH3: 9.1, pKa OH: 10.1)
Rank pKa values from low to high (2.3, 9.1, 10.1)
_ 2.3 _ 9.6 _ 10.1 _
Drop pH to well below the lowest pKa (pH = 1)
(+1) 2.3 _ 9.7 _ 10.1
At this pH, all groups will be protonated
As you raise the pH between pKa units, the molecule loses a proton & the charge goes down by 1 (thus if we jump to a pH between 2.3 and 9.7, the charge of the molecule will decrease to 0)
(+1) 2.3 (0) 9.1 (-1) 10.1 (-2)
pH 1 pH 5 pH 9.6 pH 13
pI is the avg of the 2 pKa values that are before & after 0 (2.3+9.1)/2 = 5.5
As you raise the pH between pKa units, the charge _
goes down by 1
Amino acid Strecker synthesis
R group of each functional group is the amino acid side chain)
aldehyde (formaldehyde has R group H, thus it would be used to synthesize glycine; if you wanted to synthesize alanine, R group would be CH3) >
React with NH3 / HCl (NH4Cl) >
imine intermediate (amine double bound to C, the nitrogen version of a carbonyl) >
React with HCN (or CN-) >
Intermediate with N where we want it and a nitryl group which will be hydrolyzed in the final step >
React with H3O+ (hydrolyze) >
Amino acid product with alpha carbon, alpha amine, alpha carboxyl, and amino acid side chain