3.1.11 - Electrode Potentials and Electrochemical Cells (AQA Chemistry)
0.0(0)
0.0(0)
Card Sorting
1/13
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
14 Terms
1
New cards
What happens during a redox reaction?
Electrons move from one atom to another
2
New cards
What happens when electrons move (flow)?
A charge is produced
3
New cards
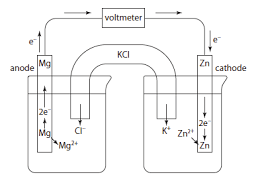
What are the metal electrodes dipped in?
A solution of their ions
4
New cards
In what direction do electrons flow?
From the negative electrode to the positive electrode
5
New cards
What is EMF or E(cell)?
The difference in voltage between two half-cells
6
New cards
What does EMF stand for?
Electromotive Force
7
New cards
What does an electrode have to be?
A solid that conducts electricity
8
New cards
What electrode would be used if a half-cell didn't contain a solid, i.e. Hydrogen?
Platinum
9
New cards
What sort of reactions occur at each electrode?
Reversible reactions
10
New cards
What does the direction of each reaction depend on?
How easily it is oxidised
11
New cards
How is oxidising ability measured and how?
Using electrode potantials, the more negative the electrode potential the more easily it is oxidised
12
New cards
What symbol do you use to separate subestances in different states?
| (single line)
13
New cards
What symbol do you use to represent the salt bridge?
|| (double line)
14
New cards
How would you write these electrochemical cells shorthand?
Mg (s) | Mg2+ (aq) || Zn2+ (aq) | Zn (s)