Strong and Weak acids
1/8
Earn XP
Description and Tags
https://www.youtube.com/watch?v=4pIHhXfGZlE&list=PL9IouNCPbCxXDlRtCQEG0cGehBvJ7t9Pf&index=10
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
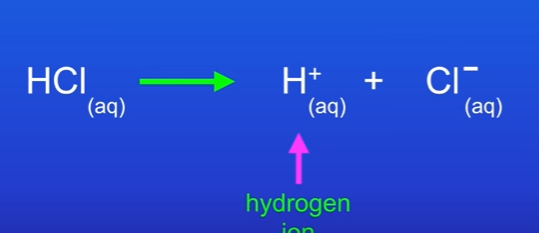
What is it called when an acid molecule splits.
We say that the acid molecule has ionised. Acid molecules ionise (split) and release H+
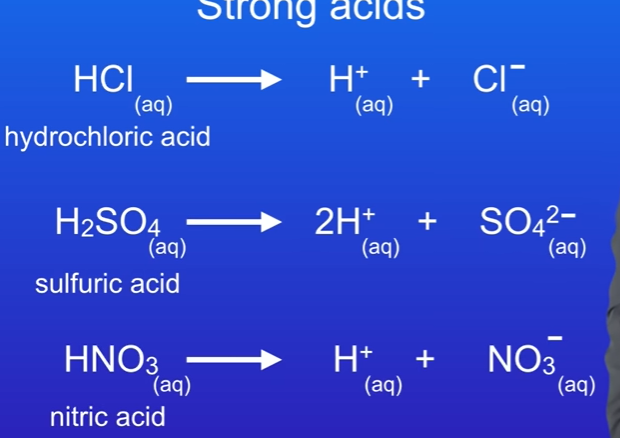
What are strong acids
They fully ionise in aqueous solutions. This can be told by the arrow, telling us the acid has fully ionised.
What are the three examples of strong acids
Hydrochloric acid, Sulfuric acid, nitric acid.
What are weak acids
They partially ionise in aqueous solutions.
The reversible reaction tells us some of the acid ionised, not all of it.
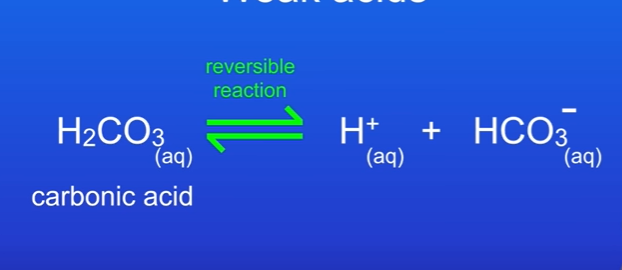
What are three weak acids
Carbonic acid
Ethanoic acid
Citric acid.
How does the pH scale give us an idea of the concentration of hydrogen ions (H+)
Strong acids have a lower pH than weak acids for a given concentration.
Thats because strong acids fully ionise, producing a greater concentration of hydrogen ions than weak acids , which partially ionise.
Whats a key fact about pH scale
As the pH scale decreases by one unit, the concentration of hydrogen ions increases by ten times.
pH 1 has a 10x greater concentration of H+ than pH 2.
pH 1 has a 100x greater concentration of h+ than pH3
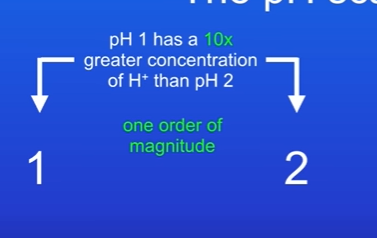
What is the 10x and 100x difference called
One order of magnitude.
The 100 x difference is called 2 orders of magnitude.
What does concentration of acid tell us
The amount of acid molecules in a given volume of solution.
A dilute acid will have fewer acid molecules in a given volume than a concentrated acid even if the strength of the acid is the same.