EXAM 2 HUMAN PHYSIOLOGY
1/124
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
125 Terms
Describe the following types of local signaling?
Autocrine > A cell releases signaling molecules that bind to receptors on the same cell.
Juxtacrine > communication that occurs between adjacent cells through direct contact with the cell next to it.
Paracrine > a cell releases signaling molecules to target nearby cells.
Synaptic > It is like paracrine but its neurons stimulate a neighboring neuron.
Describe how neurohormones & hormones are used for systemic signaling processes?
Neurohormones: Released by neurons into the bloodstream to act on distant target cells (e.g., ADH and oxytocin from the posterior pituitary).
Hormones: Secreted by endocrine glands into the bloodstream to act on distant target cells (e.g., cortisol, thyroxine).
What is the difference between a ligand and a hormone?
Ligand: Any molecule that binds to a receptor
Hormone: A specific type of ligand secreted by endocrine glands to regulate physiological processes.
Broadly describe the components of a signaling pathway.
> Recognition of a signaling molecule by a cellular receptor which activates intracellular signal molecules via second messenger molecules after target proteins create a response.
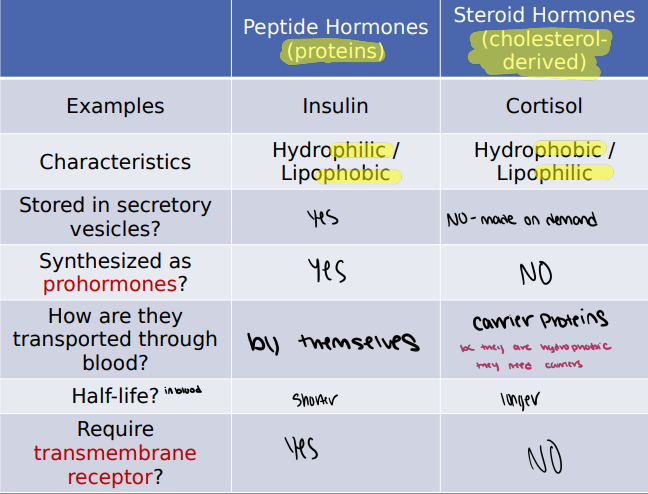
Describe the difference between cellular receptors for lipophilic and lipophobic hormones.
Lipophillic: Fat soluble, intacellular, slow, requires carrier proteins
Lipophobic: Water soluble, cell membrane, fast, dissolves in plasma, activates
How is it possible that the same signaling molecule can produce differing signaling effects in different cells?
depending on expression of receptors (Different cells may express different types or amounts of receptors)
and intracellular signaling molecules (signaling proteins and pathways activated within the cell can differ)
7. Define the term hormone and describe (in general) what a hormone does.
Hormones regulate enzymatic reactions, membrane transport, gene expression, ect.
List several characteristics which classify signaling molecules as hormones.
Secreted by endocrine glands or individual cells
Secreted into the blood for transport to distant targets
Effective at low concentrations
The same hormone can have varied effects on different tissues
Duration of action is tightly regulated by amount secreted and degradation in the blood
What types of cells and tissues secrete hormones?
Peptide, Steroid, Amino-acid derived
10. Distinguish between the three classes of hormones (peptides, steroids, and amine). Explain:
a. their chemical differences
b. their half-life
c. how they are transported in the blood
d. how they are stored
e. location of their receptors
f. their cellular mechanisms of action

Why are carrier proteins required to transport steroid hormones in the blood?
Steroid hormones are lipophilic, meaning they are insoluble in plasma (hydrophobic) and must be transported by carrier proteins to reach target tissues.
Why are peptide hormones stored in vesicles, but steroid hormones are not?
Peptide hormones are hydrophilic and can be stored in vesicles for later release however steroid hormones are lipophilic and diffuse freely across membranes so they are synthesized on demand instead of stored.
What is a prohormone? Give an example. How is a prohormone converted into a hormone?
A prohormone is an inactive precursor of a hormone that must be chopped off into its active form.
Example: Proinsulin porcesses to insulin and C-peptide
What is C-peptide, and how is it used to measure insulin production?
It is a byproduct of insulin synthesis,C-peptide is a substance produced in the body alongside insulin, in equal amounts, making it a reliable indicator of how much insulin in the body
Explain the general sequence of events that follow steroid hormones binding to intracellular receptors
Hormone diffuses through the cell membrane.
Binds to intracellular receptor.
Hormone-receptor complex translocate to the nucleus.
Binds to DNA and regulates gene transcription.
New proteins are synthesized, leading to a cellular response.
16. Describe the general sequence of events that follow peptide hormones binding to a cell surface receptor.
Hormone binds to cell surface receptor.
Receptor activates intracellular signaling pathways (often involving second messengers).
Signaling cascade leads to a cellular response (e.g., enzyme activation, ion channel opening, gene expression changes).
17. List the similarities and differences between the anterior pituitary and the posterior pituitary.
Anterior Pituitary: True endocrine gland (secretes hormones into bloodstream), epithelial origin (originates from epithelial tissue)
Posterior Pituitary: Extension of neural tissue, stores hypothalamic hormones
** All hormones secreted by the pituitary are peptide hormones
18. Why is the posterior pituitary not considered a true endocrine gland?
It does not synthesize hormones; it only stores and releases hormones produced in the hypothalamus.
List the hormones secreted by the anterior pituitary and posterior pituitary and identify the ones that have trophic effects.
Anterior Pituitary:
Growth Hormone > Trophic
Adrenocorticotropic hormone (ACTH) > Trophic
Thyroid-stimulating hormone (TSH) > Trophic
Follicle-stimulating hormone (FSH) > Trophic
Luteinizing hormone (LH) > Trophic
Prolactin (PRL)
Posterior Pituitary:
Vasopressin
Oxytocin
20. Define the role of a trophic hormone.
A hormone that stimulates the secretion of another hormone from a target endocrine gland.
21. What is a neurohormone?
A hormone produced by a neuron and released into the bloodstream.
22. How is hormone secretion in the posterior pituitary controlled?
Controlled by action potentials from neurons originating in the hypothalamus. These neurons extend their axons down the infundibulum to the posterior pituitary.
23. How do hypothalamic trophic hormones reach the anterior pituitary?
via the hypothalamic-hypophyseal portal system
24. Why are hypothalamic trophic hormones undetectable within the general circulation?
The portal system delivers hypothalamic trophic hormones directly to the anterior pituitary in a concentrated form, preventing them from being diluted in the general circulation.
25. What is a portal system? List the benefits of the hypothalamic-hypophyseal portal system.
Definition: A vascular system in which blood flows from one capillary bed to another before returning to the heart.
Benefits of Hypothalamic-Hypophyseal Portal System:
Direct delivery of concentrated hormones
Prevents dilution in systemic circulation
Allows for rapid and efficient communication between the hypothalamus and anterior pituitary
List the names of the hormones produced by the following glands and the hypothalamic trophic hormones and anterior pituitary trophic hormones which control their secretion:
Liver:
Hormone: Insulin-like growth factors (IGFs)
Hypothalamic trophic hormone: Growth hormone-releasing hormone (GHRH)
Anterior pituitary trophic hormone: Growth hormone (GH)
Adrenal cortex:
Hormone: Cortisol
Hypothalamic trophic hormone: Corticotropin-releasing hormone (CRH)
Anterior pituitary trophic hormone: Adrenocorticotropic hormone (ACTH)
Thyroid gland:
Hormones: Triiodothyronine (T3) and thyroxine (T4)
Hypothalamic trophic hormone: Thyrotropin-releasing hormone (TRH)
Anterior pituitary trophic hormone: Thyroid-stimulating hormone (TSH)
27. Describe the location and structure of the adrenal gland. Which hormones are produced by the adrenal gland?
Location: On top of the kidneys
Structure:
Adrenal cortex (outer layer): Endocrine tissue
Adrenal medulla (inner layer): Neuroendocrine tissue
Hormones:
Adrenal cortex: Cortisol
Adrenal medulla: Epinephrine
28. What are the main physiological functions of cortisol?
It promotes essential responses to stress:
Gluconeogenesis
Glycogenolysis
Lipolysis
Skeletal protein breakdown
Note negative feedback mechanisms
****a homeostatic mechanism once cortisol is relased it blocks the release of trophic hormones
29. How is cortisol secretion by the adrenal cortex controlled by the hypothalamus?
Hypothalamus releases corticotropin-releasing hormone (CRH)
CRH stimulates the anterior pituitary to release adrenocorticotropic hormone (ACTH)
ACTH stimulates the adrenal cortex to release cortisol
Cortisol exerts negative feedback on the hypothalamus and anterior pituitary
30. Describe the structure and location of the thyroid gland.
Butterfly-shaped thyroid gland lies across the trachea, inferior to the larynx
31. Which hormones are produced by the thyroid gland?
Triiodothyronine (T3 ) & Tetraiodothyronine (T4 ) or thyroxine which target many tissues
32. What are the main physiological functions of thyroxine?
Essential for normal growth/development, increases heart rate, respiratory rate, oxygen consumption, activity of metabolic enzymes, protein synthesis
33. How is thyroxine secretion by the thyroid gland controlled by the hypothalamus?
Hypothalamus releases thyrotropin-releasing hormone (TRH)
TRH stimulates the anterior pituitary to release thyroid-stimulating hormone (TSH)
TSH stimulates the thyroid gland to release T3 and T4
T3 and T4 exert negative feedback on the hypothalamus and anterior pituitary
34. Discuss the causes and physiological effects of hyperthyroidism and hypothyroidism.
Hyperthyroidism: Graves' disease
Causes: Production of thyroid stimulating antibodies which bind/activate TSH receptors on thyroid epithelial cellsitis
Physiological effects: High T3 /T4 Low TRH & TSH
Hypothyroidism: *iodine deficiency/Hashimoto's disease
Causes:
*T3/T4 cannot be synthesized by the thyroid gland
Auto-immune disorder where
antibodies attack and destroy
thyroid epithelial cells
Physiological effects: Low T3/T4 High TRH & TSH
1. Describe the major components of the central and peripheral nervous systems.
Central Nervous System (CNS):
Consists of the brain and spinal cord.
Acts as the control center, processing information.
Peripheral Nervous System (PNS):
Consists of all nervous tissue outside the CNS.
Has two divisions:
Sensory/Afferent Division: Carries signals from the periphery to the CNS (somatosensory and visceral sensory neurons).
Motor/Efferent Division: Carries signals from the CNS to the periphery (
somatic motor neurons
→ skeletal muscle contraction → E.g., arm movement
autonomic motor neurons
s → smooth muscle
contraction/relaxation → E.g.,
vasoconstriction/dilation
2. Draw a neuron and label its main parts, being sure to describe the role of each in neuronal signaling:
a. Cell body (Soma): Contains the nucleus and organelles; integrates signals.
b. Dendrites: small and numerous afferent projections, contain spines
c. Dendritic spines: Increase surface area for synapses; play a role in learning and memory.
d. Axon: long, efferent projection, branch into collaterals, end in termini
e. Axon termini (Synaptic terminals): Release neurotransmitters.
f. Axon collaterals: Branches of the axon that allow a neuron to communicate with multiple targets.
g. Axon hillock (Trigger zone): Initiates action potentials.
3. What are dendritic spines? Describe their role in memory and learning?
Dendritic spines are small protrusions from dendrites that increase the surface area available for synapses.
They play a crucial role in memory and learning by:
Providing specialized compartments for synaptic signaling.
Allowing for changes in synaptic strength (plasticity).
4. What is myelination? What type of cells make up myelin, and how does it form?
Myelination is the process of wrapping axons with myelin
Schwann cells wrap around axons to form multiple rings of cell membrane called myelin
5. What is the difference between internodes and nodes of Ranvier?
Internodes: Myelinated segments of the axon.
Nodes of Ranvier: Unmyelinated gaps between internodes, where voltage-gated ion channels are concentrated.
6. How is the concentration gradient of an ion established across the cell membrane?
Established by active transport mechanisms (e.g., the Na+/K+ ATPase pump) that move ions against their concentration gradients.
**Concentrated outside: Na+, Ca2+, Cl- higher
▪ Concentrated inside: K+, anions- lower
6. How is the concentration gradient of an ion established across the cell membrane?
Net internal negative charge
Electrochemical gradients across membranes, but the body is electrically neutral overall
7. How is the equilibrium gradient of an ion established across the cell membrane?
Net internal negative charge
Electrochemical gradients across membranes, but the body is electrically neutral overall
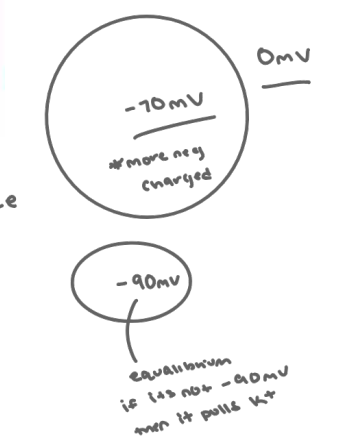
8. Define equilibrium potential of an ion and clearly describe how it is determined.
Equilibrium potential of an ion (E ion): Electrical potential difference which exactly opposes concentration gradient for a specific ion
We insert a leak channel for K+
K+ starts to move out of cell down its concentration (creating a charge difference)
The A- cannot follow K+ out of the cell because the cell is not permeable to A-
Addition K+ leaves the cell (now you have a net - charge inside the cell, the more K+ goes down it never gets to equilibrium, net + charge brings more K+ back in)
Now the negative charge inside the cell begins to attract ECF K+ back into the cell: an electrical gradient in the opposite direction from the concentration gradient.
9. Explain the components of the Nernst equation and how it is used to calculate equilibrium potential of an ion.
The Nernst equation measures the membrane potential a single ion would produce if the membrane was only permeable to that one ion.
Components include:
Gas constant (R =61)
Ion charge (z)
Ion concentrations (inside and outside the cell)
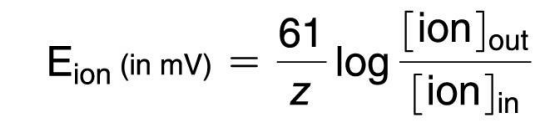
10. Define resting membrane potential and relate it to a cell’s selective permeability for specific ions/11. Why is the resting membrane potential of neurons closest to the equilibrium potential of K+ ions?
The resting membrane potential (Vm) is a measure of the electrical potential difference across the membrane created by electrochemical gradients of all ions.
It is closest to the EK because cells have many more leak channels for K (40 times more permeable to K than Na) It is maintained by the Na/K pump
*It is closest to the EK because cells have many more leak channels for K (40 times more permeable to K than Na)
11. Why is the resting membrane potential of neurons closest to the equilibrium potential of K+ ions?
12. What is the Goldman equation and what is it used to calculate?
Calculates resting membrane potential from contributions of all ions which can cross the membrane (usually Na+, K+, and Cl-) Takes into account relative permeability of the
membrane to the ions:
13. The equilibrium potential of a particular, hypothetical ion is -40mV. At this membrane potential, the ion is more concentrated inside the cell than outside the cell. Does this ion carry a positive or negative charge?
Negative Charge. Because the inside of the cell is negative, and the ion is in higher concentration inside of the cell.
14. An equilibrium potential of a hypothetical negatively charged ion is -125mV. At this membrane potential, is the ion more concentrated inside or outside of the cell?
Inside the cell. Because the equilibrium potential is negative, the negative ions are attracted to the positive outside of the cell, but are in higher concentration inside of the cell.
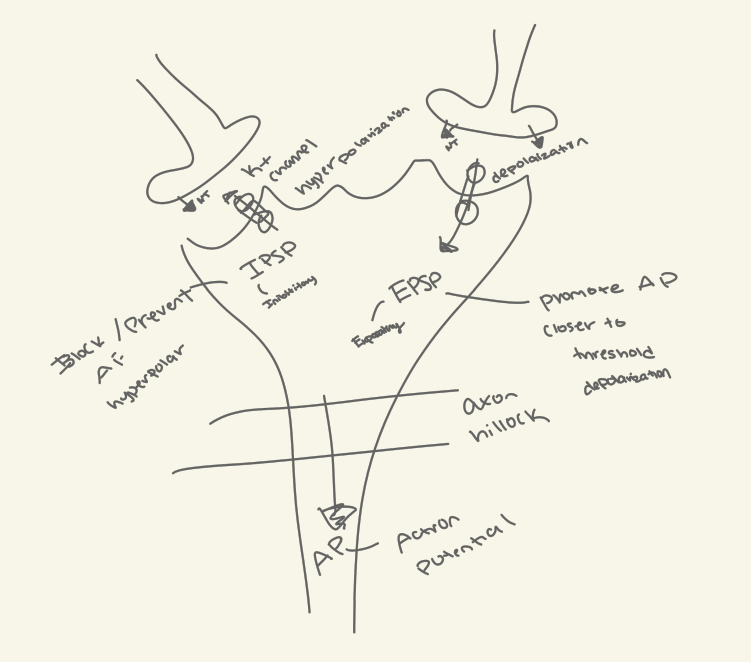
15. Compare and contrast the following terms: depolarization, repolarization, hyperpolarization
a. Depolarization: Membrane potential becomes less negative or positive.
b. Repolarization: Membrane potential returns to the resting potential.
c. Hyperpolarization: Membrane potential becomes more negative than the resting potential.
16. Diagram graded potentials and explain how they can either inhibit or initiate an action potential.????
They can:
Initiate an action potential if they are strong enough to reach the threshold at the axon hillock.
Inhibit an action potential by hyperpolarizing the membrane.
17. Which types of receptors (chemically-, mechanically- or voltage-gated?) are responsible for triggering graded potentials in post-synaptic neurons? Clearly explain your answer.
Chemically gated receptors. Neurotransmitters bind to these receptors, opening ion channels and causing a graded potential.
18. Compare and contrast suprathreshold and subthreshold graded potentials.
Suprathreshold: A graded potential starts above threshold at its initiation point but decreases in strength as it travels through the cell body. At the trigger zone, it is below threshold and, therefore, does not initiate an action potential.
Subthreshold: A stronger stimulus at the same point on the cell body creates a graded potential that is still above threshold by the time it reaches the trigger zone, so an action potential results.
19. Explain how graded potentials can be summed, and describe the difference between temporal and spatial summation.
Graded potentials can be summed to reach the threshold for an action potential.
Temporal summation: Occurs when two graded potential from one presynaptic neuron occur close together in time
Spatial summation: Multiple graded potentials from different synapses occurring at the same time.
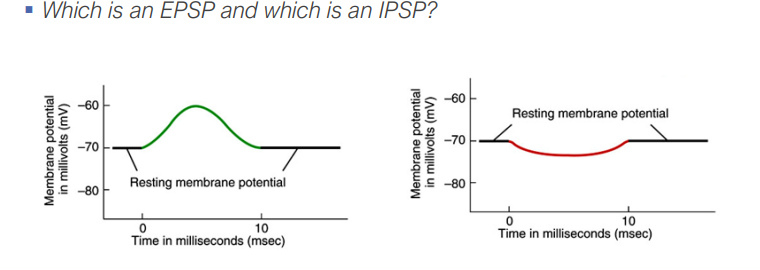
20. Clearly describe the difference between excitatory and inhibitory post-synaptic potentials, and list the different ions responsible for either potential.
EPSPs (Excitatory Post-Synaptic Potentials): Depolarize the membrane, making an action potential more likely. Ions involved: Na+, Ca2+.
IPSPs (Inhibitory Post-Synaptic Potentials): Hyperpolarize the membrane, making an action potential less likely. Ions involved: Cl-, K+.
21. What part of a neuron initiates an action potential? What transporter is present at the axon hillock in high amounts?
The axon hillock initiates the action potential.
Voltage gated Na+ channels are present in high amounts.
22. Diagram an action potential and describe how the membrane potential changes during the rising phase, the falling phase, and refractory periods of an action potential./23. Describe the roles of ions and ion channels (and their gating mechanisms) during an action potential.
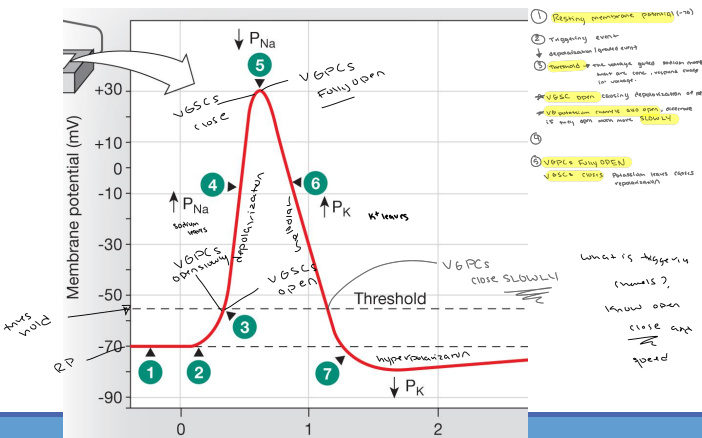
24. Why do voltage-gated sodium channels close at the height of depolarization?
They inactivate.
25. Why are action potentials not “graded”?
They are all-or-none events.
26. Could an action potential which is generated at the axon ever be conducted towards the nerve cell body? Why or why not?
No. The refractory period prevents backward propagation.
27. Could two action potentials be triggered at the same time? Why or why not?
No, due to the absolute refractory period.
28. Could the effect of two action potentials be summed? Why or why not?
No. Action potentials are all or none.
29. What is the refractory period? Why does it occur? Name the two important properties of action potentials that are a direct result of the refractory period?
30. Diagram the mechanism by which an action potential is conducted within a neuron and explain the factors contributing to signal directionality.
Graded Potential above threshold reaches the trigger zone
Voltage-gated Na+ channels open, and Na+ enters the axon
Positive charge flows into adjacent sections of the axon by local current flow
Local current flow from the active region cause new sections of the membrane to depolarize
The refractory period prevents backward conduction. Loss of K+ from the cytoplasm repolarizes the membrane.
The refractory period: As mentioned above, the refractory period prevents the action potential from traveling backwards.
The distribution of sodium channels: Sodium channels are clustered at the axon hillock and at the nodes of Ranvier. This ensures that the action potential can only travel in one direction.
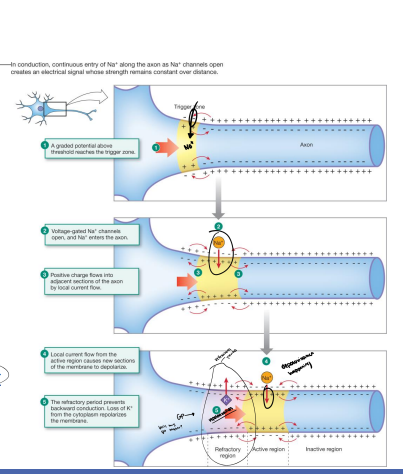
32. Explain how axon diameter and myelin affect the speed of action potentials.
Axon diameter: The larger the diameter of an axon, the faster the action potential will travel. Larger axons have less resistance to the flow of ions. (larger diameter = less resistance)
Myelin: Myelin is a fatty substance that insulates axons. This insulation prevents ions from leaking out of the axon, which allows the action potential to travel faster
33. Describe several demyelinating diseases and their effects on AP conduction.
Demyelinating diseases are a group of diseases that damage the myelin sheath. This damage can slow down or block the conduction of action potentials.
Multiple Sclerosis: MS is an autoimmune disease that attacks the myelin sheath in the brain and spinal cord.
Guillain-Barre Syndrome: GBS is a rare autoimmune disease that attacks the myelin sheath in the peripheral nervous system.
34. How do new synapses form?
35. Describe the role of growth cones and chemotaxis in the process of synapse formation.
Growth cones are specialized structures at the tips of axons that guide the axon to its target cell. (ends of neurons that aren’t migratory)
Chemotaxis is the process by which cells are attracted or repelled by chemical signals. Growth cones use chemotaxis to find their way to their target cells.
36. When referenced to synapse formation, what does “use it or lose it” refer to?
The phrase "use it or lose it" refers to the fact that synapses that are not used are more likely to be eliminated. This process is known as synaptic pruning. Synaptic pruning is a normal part of brain development. However, it can also occur in adulthood in response to experience.
37. Compare and contrast:
a. Chemical and Electrical Synapses
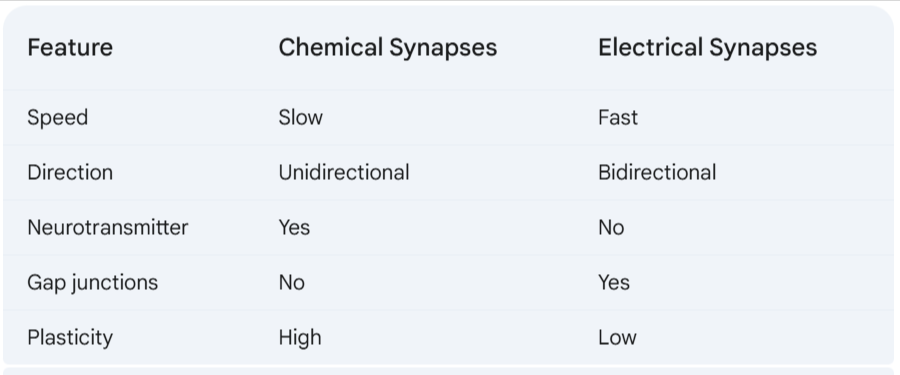
C&C
b. Neuronal and Neuromuscular Synapses

C&C
c. Neurotransmitters and Neuromodulators
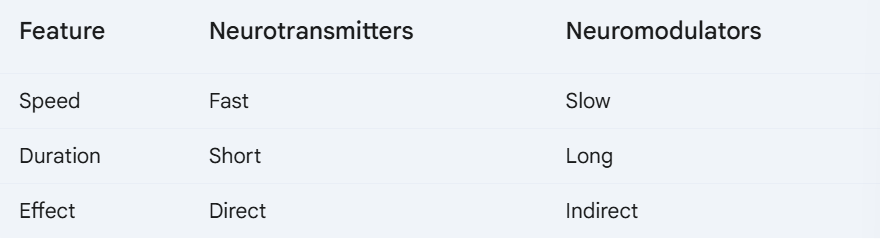
C&C
d. Ionotropic & Metabotropic receptors
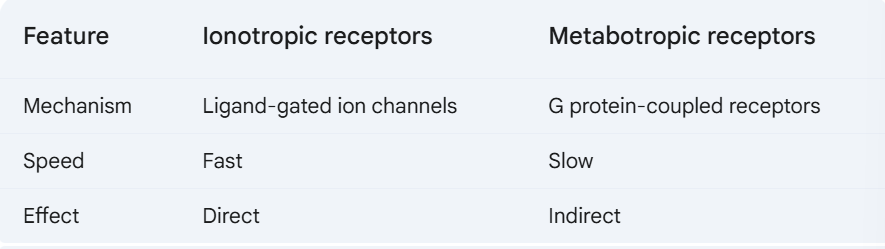
C&C
e. Cholinergic and Adrenergic receptors
C&C
f. Nicotinic and Muscarinic receptors
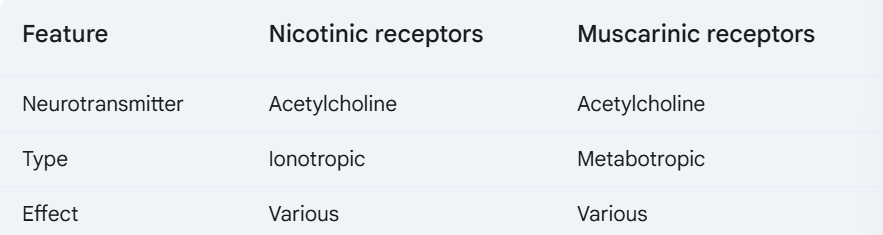
38. Cleary describe the mechanism by which neurotransmitters are released into the synaptic cleft, and their effect on post-synaptic cells.
Neurotransmitters can be returned to axon terminals for reuse or transported into glial cells
Enzymes inactivate neurotransmitters
Neurotransmitters can diffuse out of the synaptic cleft
39. Cleary describe the mechanisms by which neurotransmitters are cleared from the synaptic cleft. Use the clearance of acetylcholine as a specific example.
Acetylcholine is made from choline and acetyl CoA
In the synaptic cleft Acetylcholine is rapidly broken down by the enzyme acetylcholinesterase
Choline is transported back into the axon terminal by cotransport with Na+
Recycled choline is used to make more Acetylcholine
40. Describe the structure of gap junctions and their main function in cells.
Gap junctions are intercellular channels that allow ions and small molecules to pass between cells. They are made up of six connexin proteins that form a hemichannel. Two hemichannels from adjacent cells come together to form a gap junction.
2 hemichannels (connexons) = 1 gap junction (hydrophilic pore)
Each connexon = 6 connexins
The main function of gap junctions is to allow cells to communicate with each other. They are particularly important in excitable tissues such as the heart and nervous system.
41. Why does neuronal signaling occur via both chemical and neuronal synapses? Explain the benefits of each.
Neuronal signaling occurs via both chemical and electrical synapses because each type of synapse has its own benefits.
Chemical synapses are slower than electrical synapses, but they are more versatile. Chemical synapses can be excitatory or inhibitory, and they can be modulated by a variety of factors.
Electrical synapses are faster than chemical synapses, but they are less versatile. Electrical synapses are always excitatory
42. Explain the differences in how ionotropic and metabotropic receptors trigger EPSPs or IPSPs.
Ionotropic receptors are ligand-gated ion channels. When a neurotransmitter binds to an ionotropic receptor, it opens the channel, allowing ions to flow into or out of the cell. This can cause an EPSP (excitatory postsynaptic potential) or an IPSP (inhibitory postsynaptic potential).
Metabotropic receptors are G protein-coupled receptors. When a neurotransmitter binds to a metabotropic receptor, it activates a G protein. The G protein then activates an enzyme that produces a second messenger. The second messenger can then open or close ion channels, or it can activate other enzymes that can modify the cell in other ways. Metabotropic receptors can also cause EPSPs or IPSPs, but they do so through a more indirect mechanism than ionotropic receptors.
43. List the main functions of the following neurotransmitters:
a. Acetylcholine
b. Dopamine
c. Serotonin
d. Norepinephrine
Acetylcholine: Acetylcholine is a neurotransmitter that is involved in a variety of functions, including control of skeletal/smooth muscle, glands
Dopamine: Dopamine is a neurotransmitter that is involved in movement, motivation, and reward.
Serotonin: Serotonin is a neurotransmitter that is involved in mood, sleep, and appetite.
Norepinephrine: Norepinephrine is a neurotransmitter that is involved in control of smooth muscle, glands
44. Name a type of inhibitory neurotransmitter and describe how it functions.
GABA (gamma-aminobutyric acid) is a type of inhibitory neurotransmitter. When GABA binds to its receptor, it opens chloride channels. This allows chloride ions to flow into the cell, which makes the cell more negative. This makes it more difficult for the cell to fire an action potential.
45. How do the following neurotoxins interfere with neuronal function?
a. Botulinum toxin
b. Black widow venom
c. Nicotine
d. Morphine
Botulinum toxin: blocks acetylcholine release
Black widow venom: Increase in intracellular [Ca] and neurotransmitter release
Nicotine: mimics acetylcholine by binding to nicotinic cholinergic receptors
Morphine: blocks neurotransmitter release at pain synapses
1. Clearly describe how neurons are bundled into fascicles and nerves.
Bundle of neurons wrapped with connective tissue = fascicle
Bundle of fascicles wrapped with connective tissue = nerve
2. Discuss how the following parameters affect the speed of action potentials:
a. internal resistance
Ions moving through the cytoplasm of a neuron experience friction, which slows down the speed of action potential propagation. This is called 'internal resistance'.
The diameter of a neuron affects the internal resistance. A larger diameter means there will be less internal resistance, and therefore a higher current and faster action potential.
b. membrane resistance
Ions can also experience friction as they pass across a neuron's membrane. This is called 'membrane resistance'.
If a neuron is myelinated, it will have a higher membrane resistance, and therefore a higher current and faster action potential.
3. Why do large diameter myelinated axons have the fastest action potentials?
A large diameter axon experiences less internal resistance, and therefore has a higher current and faster action potential.
A myelinated axon experiences higher membrane resistance, and therefore a higher current and faster action potential.
Since both of these work to increase current, a large diameter myelinated axon will have the fastest action potential.
5. How does a CAP differ from a normal action potential?
A normal action potential is the all-or-nothing change in membrane potential of an individual cell (a neuron or muscle). A CAP, on the other hand, represents the summed electrical activity of multiple cells within a nerve or muscle.
6. Clearly explain why the amplitude of a CAP can be changed, but the amplitude of an AP cannot.
A CAP is the sum of multiple action potentials. Because it represents the combined activity of many cells, its amplitude can vary depending on the number of cells activated and their individual contributions. In contrast, an action potential is an all-or-nothing event that occurs in a single cell, so its amplitude is constant.
7. Explain how the following two parameters are measured:
a. mean nerve conduction velocity (MNCV)
b. CAP amplitude
a. mean nerve conduction velocity (MNCV)
The mean nerve conduction velocity (MNCV) is a measurement of the speed at which an electrical impulse travels along a nerve. It is typically around 60 meters/second, but in this lab you may observe 10 m/sec.
The mean nerve conduction velocity (MNCV) can be calculated as the difference in distance between two recording electrodes divided by the difference in time it takes for the electrical impulse to reach those two electrodes.
b. CAP amplitude
CAP amplitude is a measure of the strength of the compound action potential. It reflects the number andsynchronization of individual nerve fibers that are activated in response to a stimulus. It is calculated as the difference in voltage between the peak of the CAP and the baseline voltage.
Description of the anatomical/functional parts of the CNS:
a. Major parts of the brain and spinal cord: The major parts of the brain include the cerebrum, cerebellum, diencephalon, and brain stem. The spinal cord is divided into cervical, thoracic, lumbar, sacral, and coccygeal segments.
b. Meninges: The meninges are protective tissue layers surrounding the brain and spinal cord.
c. Bones protecting the CNS: The cranium protects the brain, and the vertebral column protects the spinal cord.
Glial cells and their functions: Glial cells are cells in the CNS and PNS.
a. Ependymal cells: Ependymal cells create barriers between compartments and are a source of neural stem cells.
b. Astrocytes: In the CNS, astrocytes take up K+, water, and neurotransmitters, secrete neurotrophic factors, help form the blood-brain barrier, and provide substrates for ATP production.
c. Microglia: Microglia are modified immune cells that act as scavengers.
d. Oligodendrocytes: Oligodendrocytes form myelin sheaths in the CNS.
Blood-brain barrier diagram and explanation:
The blood-brain barrier is a physical barrier formed by astrocyte foot processes and endothelial tight junctions.
It controls the movement of substances by preventing solutes from crossing into the brain from the blood.
Astrocytes are involved in the formation of the blood-brain barrier through their foot processes.
Blood-brain barrier problem for drug delivery:
The blood-brain barrier poses a major problem for drug delivery because it prevents many solutes from crossing from the blood into the brain.
Layers of meninges and their functions
a. Dura mater: A thick connective tissue layer.
b. Arachnoid membrane: A fluid-filled space.
c. Pia mater: A thin epithelium layer.
The meninges, in general, provide protection and nutrition/blood supply to the brain.
Sub-arachnoid space and its contents:
The sub-arachnoid space is a fluid-filled space located between the arachnoid membrane and the pia mater. It is filled with cerebrospinal fluid (CSF).
Ventricles of the brain:
The brain contains ventricles I-IV, which are hollow, contiguous lumens.
Connection of ventricles to the central canal:
The ventricles are contiguous with the central canal in the spinal cord.
Production of cerebrospinal fluid by the choroid plexus
he choroid plexus, located on the walls of the ventricles, produces cerebrospinal fluid (CSF). It pulls fluid from arteries to produce CSF in the ventricles.
Main functions of cerebrospinal fluid:
The main functions of cerebrospinal fluid include providing physical protection (cushioning and lack of compression) and waste clearance.