RX523: Module 3
1/135
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
136 Terms
The individual tissues/organs in the body may contain a different concentration of the drug due to what?
numerous physiological and physiochemical properties and pharmacokinetic rate processes taking place
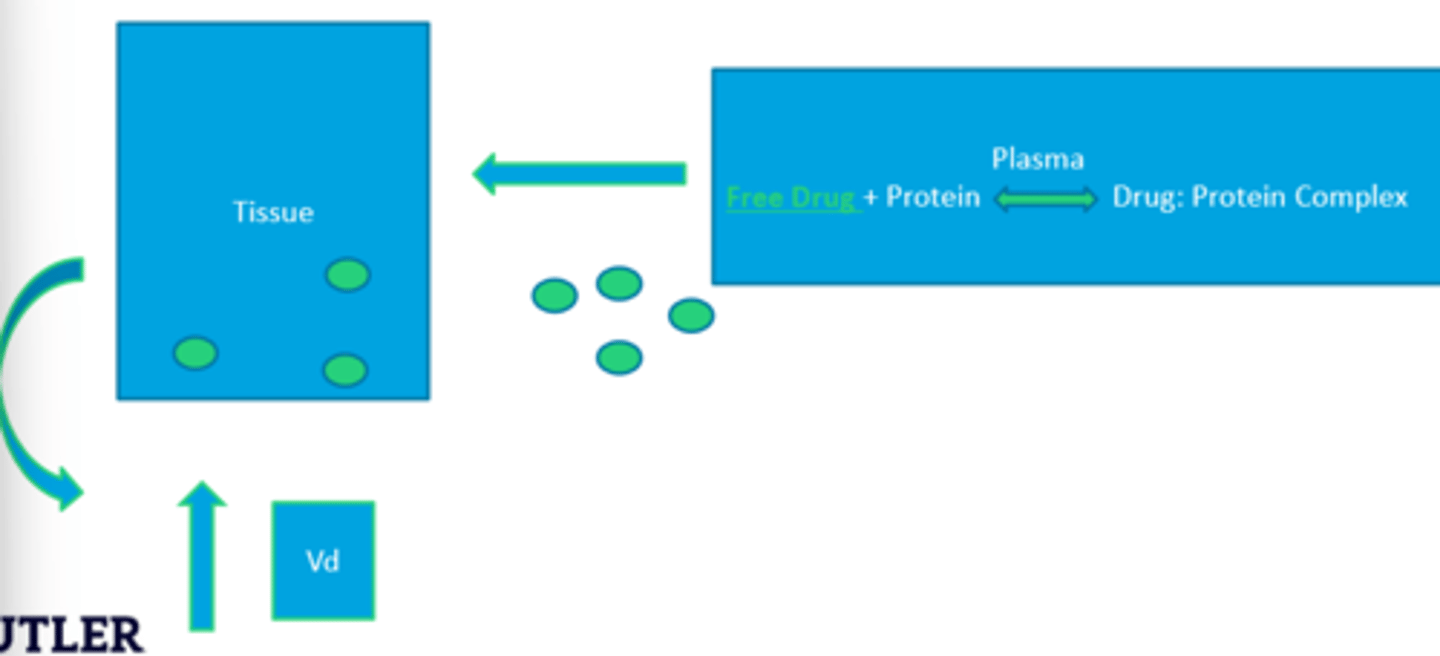
Applying Volume to Drug Distribution
The amount of drug (mass/amount of drug) in a given location can be related to observed plasma drug concentration by a proportionality constant that reflects the apparent volume of fluid into which the drug is dissolved
Apparent Vd
The apparent volume in which the drug is dissolved
Volume of distribution (Vd) is an indicator of what?
the extent of drug distribution into the body fluids and tissues
Vd relates what?
the amount of drug in the body to the measured concentration of the drug in plasma (Cp)
Vd represents what?
the result of dynamic drug distribution between the plasma and the tissues and accounts for the mass balance of the drug in the body
Vd does not represent what?
an actual physiologic compartment, organ, or tissue in the body
Apparent volume of distribution
the theoretical volume into which the drug uniformly distributions immediately following injection into the body
Vd does not indicate into what?
which specific fluids and tissues the drug distributes
Apparent Vd Considerations
- The Vd represents the size of a compartment necessary to account for the total amount of drug in the body if it were present throughout the body at the same concentration found in the plasma
- The actual physiological sites into which the drug distributes cannot be determined by the Vd; without specific information the actual sites are speculative
- The apparent volume of distribution is a function of the lipid versus water solubility of the drug, the plasma and/or tissue binding properties of the drug, and the fraction of unbound drug available for distribution
Apparent Vd is a ___ Constant
Proportionality
Apparent Vd is a Proportionality Constant
- The apparent volume of distribution is recognized as a proportionality constant and is usually a property of the drug rather than a physiological system
- The apparent volume of distribution is the proportionality constant between the Cp0 or Cmax/peak and drug dose
- The Vd is considered a constant value for each drug and remains uninfluenced by the dose administered or the route of administration
- The higher the apparent Vd, the greater is the extent to which the drug is distributed in the body tissues/organs
Calculating the Apparent Volume of Distribution (Vd): IV Bolus Model
- Observe the Cp0 from semi-logarithmic plasma drug concentration versus time curve graph and/or concentration data and calculate the Vd using the IV bolus drug dose administered
- Back-extrapolate the Cp0 following the determination of the ke using two drug concentrations obtained during drug elimination and calculate the Vd using the IV bolus drug dose administered
The Quantification of Vd: A Visual Example (image)
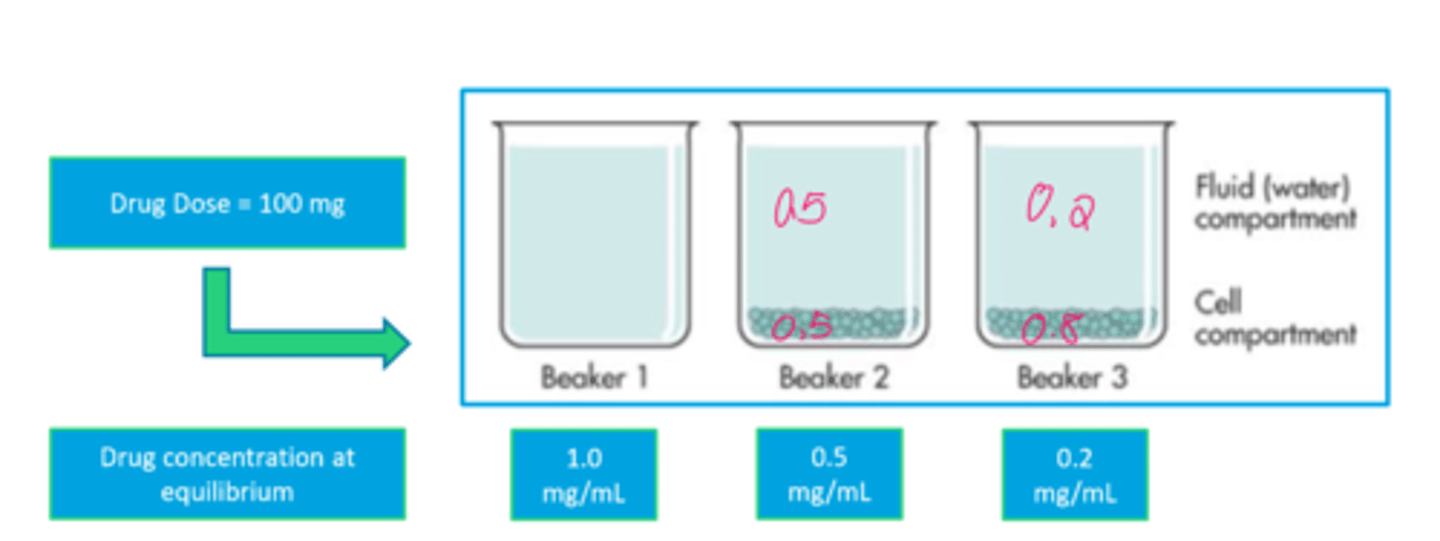
Factors Affecting the Quantification of the Apparent Vd
- Amount of drug in the cells increases
- Volume of distribution increases
Additional Factors Impacting the Magnitude of the Apparent Vd: Smaller Vd
- increasing Cp
- High water solubility
- Increased plasma protein binding
- Decreased tissue binding
Additional Factors Impacting the Magnitude of the Apparent Vd: Larger Vd
- decreasing Cp
- High lipid solubility
- Decreased plasma protein binding
- Increased tissue binding
Apparent Vd Units of Measurement
The units of measurement for apparent Vd can either be expressed as a volume (L) or a volume per patient weight (L/kg)
Apparent Vd Units of Measurement: When expressing the unit of measurement as a volume per patient weight, the weight utilized for the calculation may do what?
vary depending on the drug in question
Typically, drug dosing is based on the patient's actual body weight; however certain drugs require the use of ideal body weight or an adjusted or body weight. The weight used in the apparent Vd calculation will be the ___ used for drug dosing
weight
How to Appropriately Interpret & Utilize Vd Data
- Drug must achieve distributional equilibrium in the system before any drug concentration is measured
- Drug binding distorts the true physical volume of distribution when all components of the system are not assayed; extravascular binding increases the apparent Vd
- Both intravascular and extravascular binding must be considered to determine a meaningful Vd
- Apparent Vd is a measure of relative extent of drug distribution
- A large apparent Vd may be indicative of greater tissue binding and a smaller Vd may be indicative of greater plasma protein binding
- Relating the total mass of drug to the drug concentration and determining Vd is important in pharmacokinetic practices
Connecting the Apparent Vd to Loading Doses
- The initial plasma drug concentration (Cp0) or Cmax/Cpeak is dependent upon the size of the bolus/loading dose and the volume of distribution
- A loading dose is often administered prior to a continuous infusion or multiple intermittent infusion regimen to increase the plasma drug concentration and achieve a concentration within the therapeutic range
- The loading dose (LD) is typically larger than a normal maintenance dose
- A LD can be calculated using a desired plasma drug concentration and the known volume of distribution
Additional Factors to Consider for LD Calculations
- The apparent Vd accounts for the measurement of the drug in the body and is important in the estimation of a loading dose necessary to achieve a desired plasma drug concentration within the therapeutic range
- Additional factors to consider in the estimation of LD include the amount of drug available for distribution
Loading Dose Equation
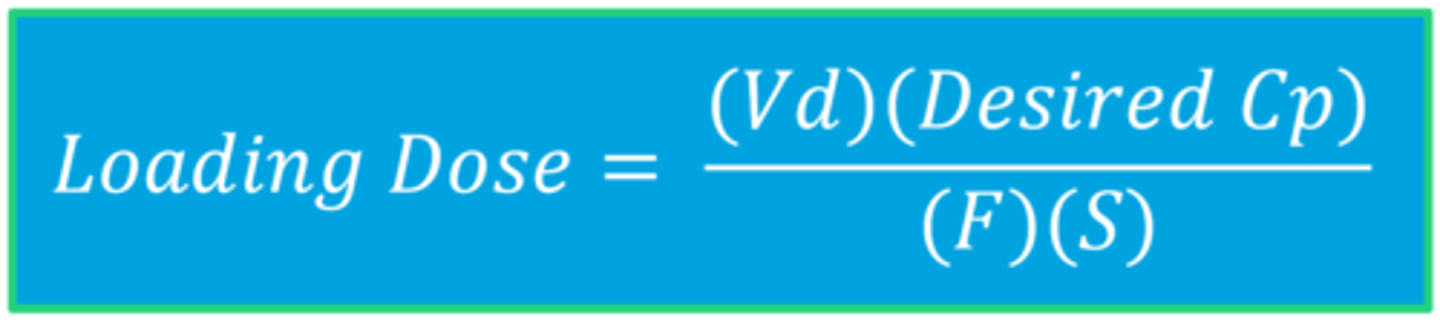
The Complete LD Calculation
- Calculation of LD with the incorporation of bioavailability and salt form
- Incorporation of the drug reaching the systemic circulation takes into account the unchanged drug reaching the systemic circulation (bioavailability) and the fraction of active drug (S)
- The inclusion of "S" attempts to correct for inert ingredients in a dosage form that may potentially impact the calculated plasma drug concentration
A loading dose of digoxin is requested for a 57-year-old male with chronic atrial fibrillation and heart failure. The patient weighs 70 kg. The prescriber desires a plasma concentration of 0.7 ng/mL. The S value = 1 and F = 0.7. The volume of distribution is 7 L/kg. Determine the appropriate loading dose and recommend the appropriate tablets to administer as whole tablet(s) given the availability of 62.5, 125, 187.5, and 250 mcg tablets.
Two 250mg tablets
Key Points to Remember Regarding Apparent Vd
- Recall the physiological and physiochemical factors that affect distribution as they impact the quantification of Vd
- Protein binding will impact Vd and those considerations will be reviewed next!
- Numerous disease states will alter drug distribution factors, including total body water and will impact the quantification of Vd and loading dose calculations
- Consider all factors that may lead to kinetic variability
The KEY Factor to Drug Distribution =
FREE DRUG
Many drugs interact with plasma or tissue proteins or other macromolecules to form what?
complexes.
Drug-protein binding is a rapidly reversible process involving what?
association and dissociation between the drug and protein
Reversible drug-protein binding implies what?
a weaker chemical bond between the drug and protein molecule
The drug-protein complex has limitations regarding what?
drug movement in the body and pharmacodynamic activity
Drug that is bound to protein cannot easily traverse cell membranes and is limited in what?
in its distribution
Drug bound to protein is generally considered what?
pharmacologically inactive
Percent protein binding equation

Factors that Affect Protein Binding
- Drug concentration
- Affinity of the protein for the drug
- The concentration of the circulating protein
- The number of binding sites available
- Presence of disease or altered physiologic state
- Presence of other protein bound drugs
- Physiochemical properties of the drug determine a drug's protein-binding characteristics
Free (Unbound) Drug Vs. The Complex (image)
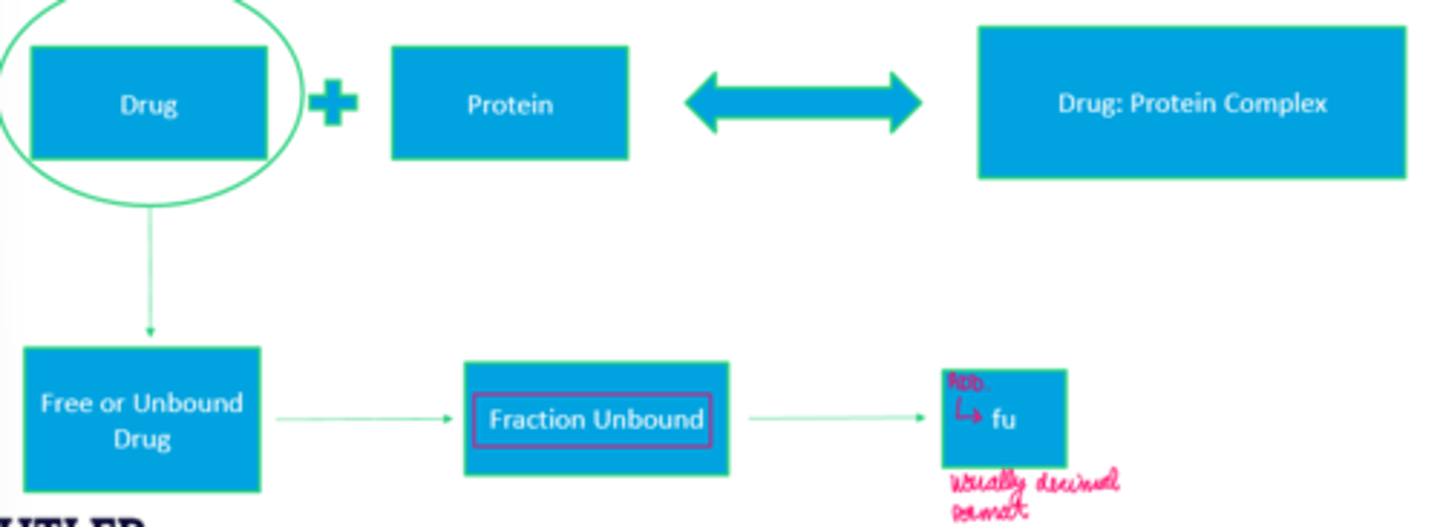
fu
Fraction Unbound
fu equation
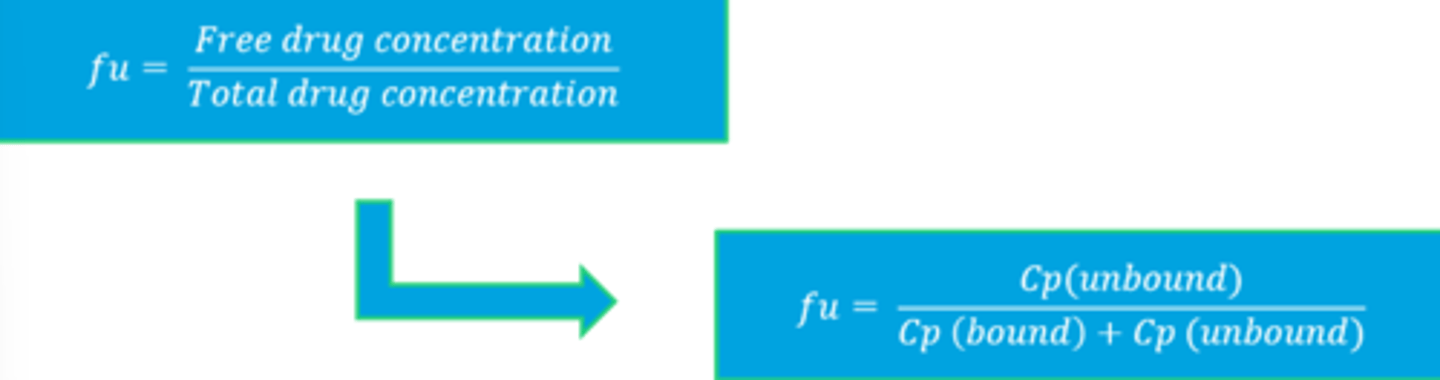
Most clinical laboratory reports of drug plasma concentrations will represent what?
drug that is both bound to plasma proteins plus the drug that is unbound (free)
The Major Proteins Involved in Drug Binding
- Albumin
- α-1 acid glycoprotein (AAG)
- Lipoproteins
- Immunoglobulins
Albumin is responsible for what?
maintaining osmotic pressure of the blood and for the transport of endogenous and exogenous substances
What is the major plasma protein responsible for reversible drug binding?
Albumin
Albumin tends to bind drugs designated as what?
weak acids
Plasma concentrations of albumin are affected by what?
a number of physiologic changes and disease states
AAG
Alpha-1 Acid Glycoprotein
Alpha-1-acid glycoprotein (AGP or AAG) is also known as what?
orosomucoid
Alpha-1-acid glycoprotein is what?
an acute phase plasma alpha-globulin glycoprotein responsible for transport of basic or neutrally charged lipophilic compounds
AAG tends to bind drugs designated as what?
weak bases
Plasma concentrations of AAG are affected by what?
a number of physiologic changes and disease states
Lipoproteins are what?
Macromolecule complexes of lipids and proteins and are classified according to their density and separation in ultracentrifuge and are responsible for transporting plasma lipids to the liver
When may Lipoproteins bind drugs?
if binding sites of albumin become saturated
Immunoglobulins (α, β, and γ) are what?
Synthesized by the liver (α, β)or manufactured by the immune system (γ)
Immunoglobulins (α, β, and γ) are responsible for what?
the plasma transport of endogenous substances
Erythrocytes may bind what?
endogenous and exogenous substances
Erythrocytes Drug binding does not typically lead to what?
a significant alteration in volume of distribution
Factors That Decrease Plasma Protein Concentration
- Decreased protein synthesis
- Increased protein catabolism
- Altered distribution
- Increased protein elimination
Conditions Leading to Increased Albumin
- Age
- Burns
- Liver disease (cirrhosis)
- Malnutrition
- Renal disease (end-state renal disease, nephrotic syndrome)
- Pregnancy
- Surgery
- Stress
- Trauma
Conditions Leading to Increased AAG
- Nephrotic syndrome
- Oral contraceptives
Conditions Leading to Increased Lipoprotein
- Hyperthyroidism
- Injury
- Trauma
Conditions Leading to Decreased Albumin
- Age
- IBD
- Myocardial infarction
- Renal failure
- Rheumatoid arthritis
- Stress
- Surgery
- Trauma
- Cancer
Conditions Leading to Decreased AAG
- Exercise
- Hypothyroidism
- Psychosis
- Schizophrenia
Conditions Leading to Decreased Lipoprotein
- Diabetes
- Hypothyroidism
- Nephrotic syndrome
A decrease in plasma protein concentration will lead to a [increase/decrease] in the amount of drug bound (Cp bound); however, the overall concentration of free/unbound drug is generally unaffected
decrease
The fraction of drug that is free (fu) increases as the plasma protein concentration [increases/decreases]
decreases
Alterations to fu and the Apparent Vd (image)
Decreased Plasma Protein Concentration example (image)
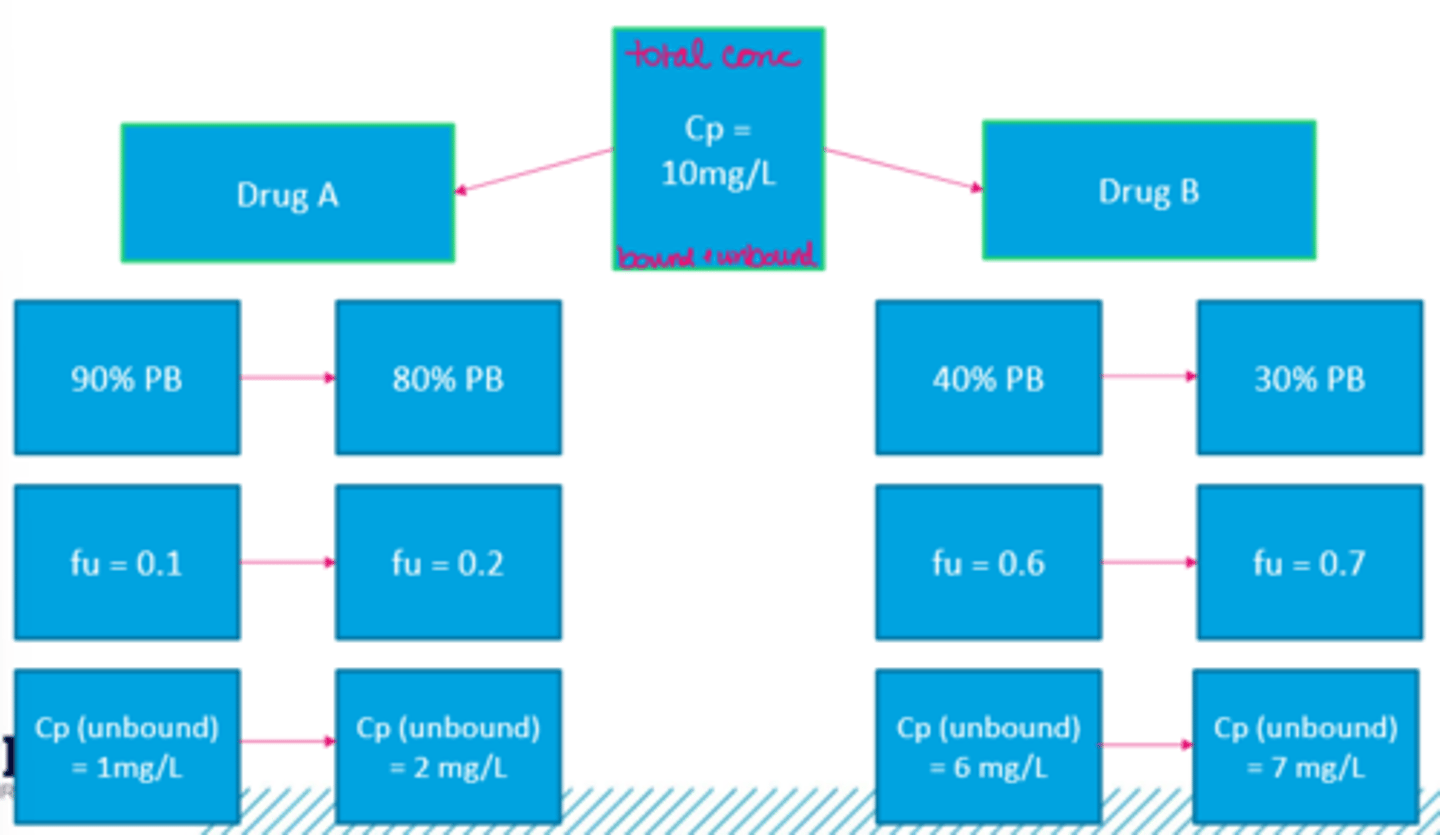
Recall that most clinical laboratories will report the total drug plasma concentration, which will include both...
free/unbound drug and drug that is bound to proteins
Alterations to the plasma protein concentration must be considered in the interpretation of reported plasma drug concentration, particularly for what?
highly bound drugs with narrow therapeutic ranges
The plasma drug concentration in a state of normal binding of albumin can be expressed utilizing the following equation (image)
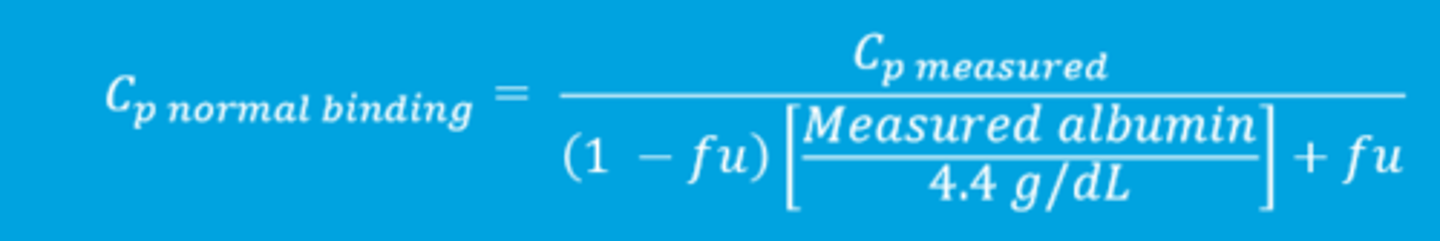
The binding affinity of plasma proteins for a drug can alter what?
the fraction of free/unbound drug
The plasma proteins in patients with uremia have [more/less] affinity for phenytoin compared to patients without uremia
less
The plasma protein binding of phenytoin is reduced and the fraction unbound (fu) is [increased/decreased]
increased
The increase in the fu would result in an [increase/decrease] in the apparent Vd as more of the drug is now free to further distribute and potentially lead to enhance pharmacodynamic effect/toxicity
increase
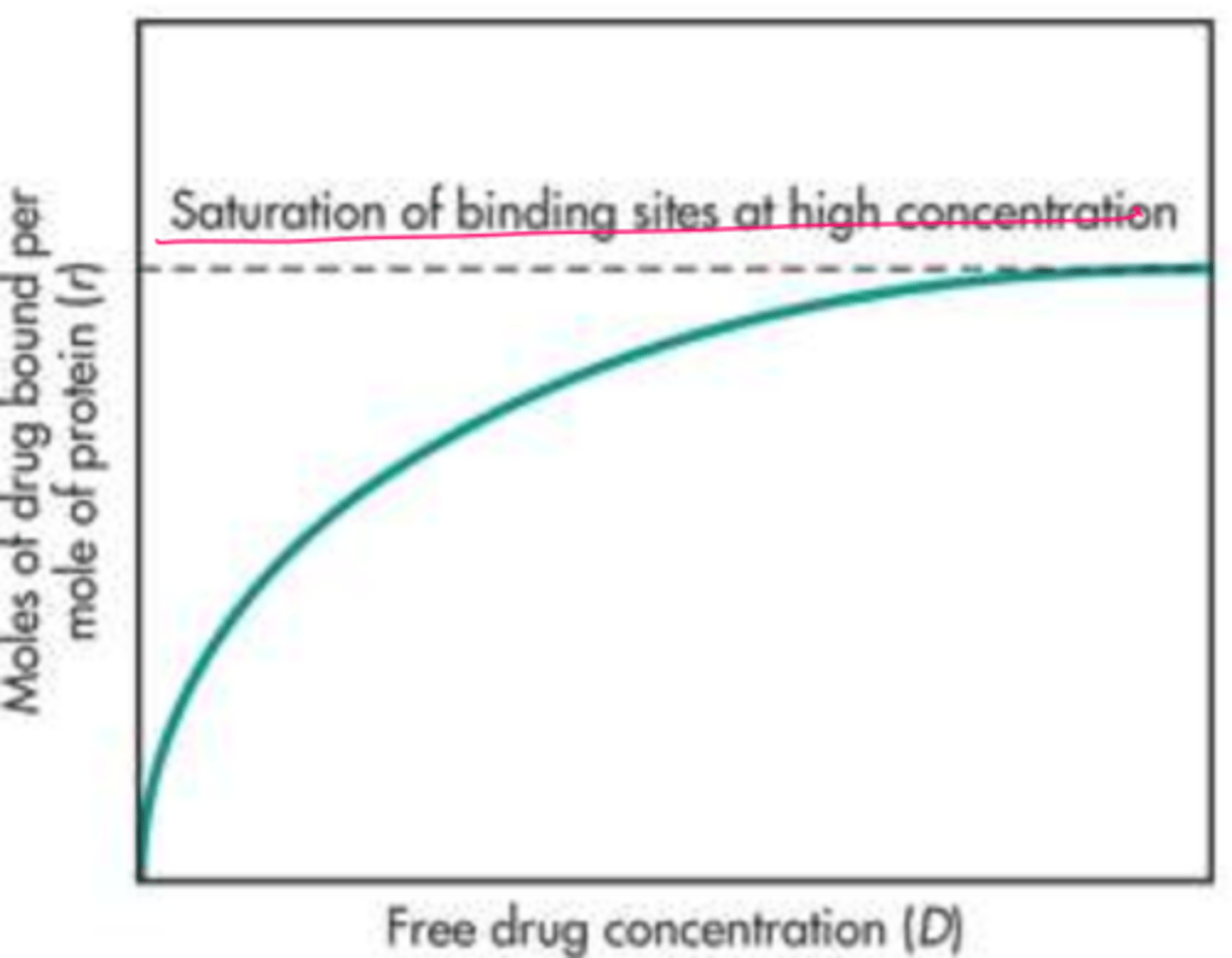
Does the fraction of free/unbound drug (fu) vary too significantly with an increase in drug dose for most drugs that are bound to albumin?
It does not
However, when drug doses result in a plasma drug concentration 25 to 50 mg/L, what may happen?
the binding sites can become saturated and then the fu will change with the increase in plasma drug concentration
Protein Binding Saturation (image)
One drug may displace a second drug bound to the protein, which will lead to an [increase/decrease] in the fraction free/unbound (fu) of the displaced drug
increase
The increase fu may lead to enhanced pharmacodynamic effects and [increase/decrease] the potential for drug toxicity
increase
What is the association with fu and Vd?
It is a positive correlation, if one is increasing, other one is increasing
Clinically significant alterations to protein binding must be critically evaluated, in particular for what kind of drugs?
For drugs with narrow therapeutic ranges and displaying high levels of protein binding (≥90%) and therefore a typical fu of ≤ 0.1
Altered PB associated with drugs displaying high levels of protein binding may lead what?
clinically significant alterations in the pharmacodynamic effect or toxicities observed
Drug Elimination
Irreversible drug removal from the body results from a combination of interconnected processes aimed at removing drug from the body
Elimination involves what?
several complex rate processes and potential organs/systems
Elimination is typically divided into two major components
biotransformation and excretion
What are the two major organs responsible for drug elimination from the body?
The liver and the kidney
What may impact the body's ability to perform drug elimination processes?
Several physiologic alterations, disease states, and drugs
Drug Elimination Processes
Drug Elimination
- Biotransformation
--- Drug is chemically converted to a metabolite
--- Liver, kidney, lung, GI tract, and skin
- Excretion
--- Removal of the drug from the body
--- Kidney, bile, sweat, saliva, milk, and lungs
Clearance (Cl)
a pharmacokinetic term that describes drug elimination from the body
Drug clearance represents a way to what?
Quantify drug elimination
Clearance considers all processes involved in drug elimination regardless of what?
the mechanism
Clearance describes what?
volume of fluid removed of the drug per unit of time
Clearance is what?
the removal of drug from a volume of plasma/blood in a given time
Clearance does not indicate the amount of drug, but rather what?
the theoretical volume of plasma/blood from which the drug is completely removed in a given time
The body is a space that contains an apparent volume of fluid (Vd) in which drug is dissolved, and clearance is what?
the fixed volume of that fluid that is removed of drug per unit of time
Units of measurement for clearance are expressed as what?
volume per unit of time (e.g. L/hr)
The total body clearance represents what?
the sum of all of the different clearance processes in the body that are occurring in parallel
General clearance equation for total body clearance (Clt)
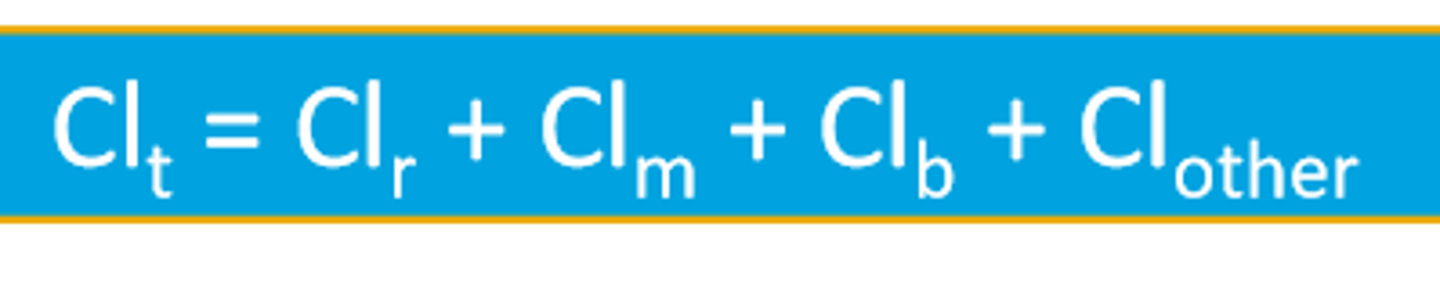
Similarly, the elimination rate constant represent the total sum of all the different rate constants for drug elimination
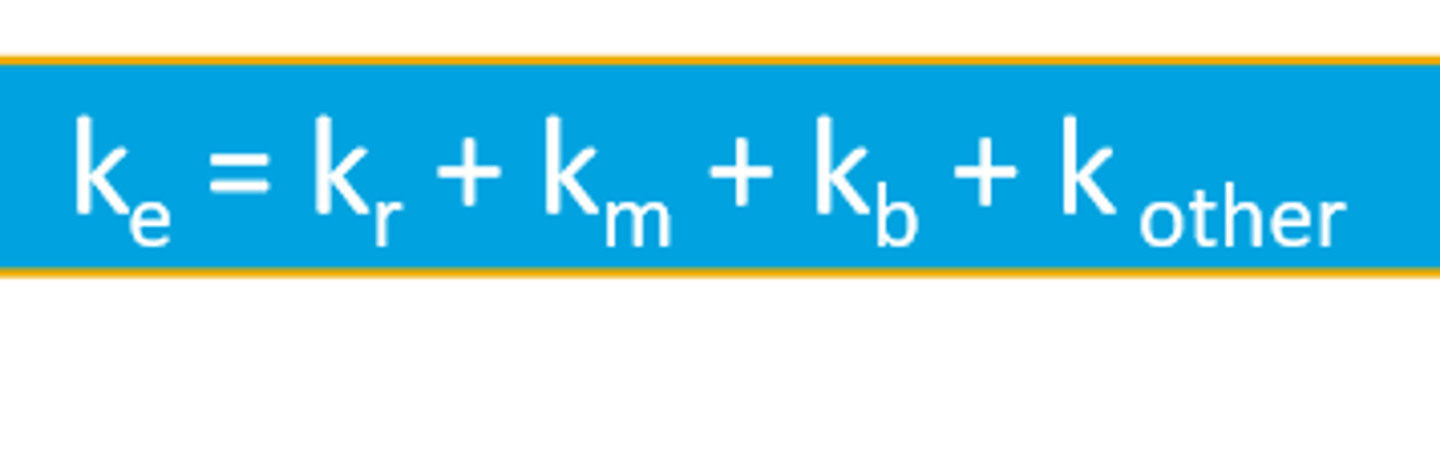
Clearance Calculation Models
1. Compartmental model
2. Physiologic model
3. Noncompartmental approach