CHAPTER 3: IONIC AND COVALENT COMPOUNDS CPQ
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
52 Terms
What is the BEST name for the compound SF6, a potent greenhouse gas? |
sulfur hexafluoride
A cation has __________ electrons and is ________ charged. |
lost; positively
Iron(II) is an example of a(n) ________. |
cation
How many valence electrons does chlorine have?
7
Below are two charged particles. What will they do when in close proximity to one another?

They will attract one another.
What is the name of CaCO3, a common calcium supplement and antacid?
calcium carbonate
Lead(II) is especially harmful to young children because it_________. |
harms brain development
In an ionic compound |
cations and anions are attracted to one another.
Carbon tetrafluoride is a refrigerant and potent greenhouse gas. Which of the following is the molecular formula for this compound?
CF4
Another name for an ionic compound is a(n) ________. |
salt
Which of the following is the formula for an ionic compound called iron(III) oxide?
Fe2O3
Which of the following is a diatomic element?
N2
NaCl
Li2O
CO
H2O
N2
An ion of fluorine has ten electrons total. What is the charge of the fluorine ion?
-1
In the methane molecule (complete the sentence)
carbon is very unstable.
carbon does not have an octet of electrons.
carbon has donated four electrons to hydrogen, leaving it with a charge of +4.
carbon is sharing its four electrons with the hydrogens.
hydrogen has eight electrons in its valence shell.
carbon is sharing its four electrons with the hydrogens.
Below are two ions (not drawn to scale). What will they do when in close proximity to one another?

They will attract one another, forming an ionic compound.
How many electrons are shared by the circled carbon and oxygen of acetone?
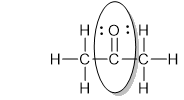
4
The total number of valence electrons in methane is
8
Which of the following Lewis dot structures best represents the chlorine atom?
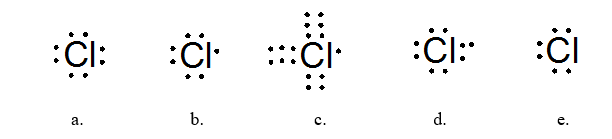
structure b
Which of the following describes 16O2–?
Protons | Neutrons | Electrons | |
a. | 8 | 8 | 10 |
b. | 8 | 8 | 6 |
c. | 16 | 16 | 14 |
d. | 16 | 16 | 16 |
e. | 6 | 12 | 8 |
choice a
What is the charge on iron in the ionic compound FeCl3? |
+3
______ is a waste product excreted by the kidneys. It is a product of the breakdown of proteins.
Urea
Which statement BEST describes the meaning of an expanded octet?
"Expanded octets" is a term that describes atoms that have more than eight electrons in their valence shells.
"Expanded octets" is used to describe elements that do not normally form an octet when bonded and would therefore have to expand to form the octet.
"Expanded octets" is used to describe how atoms accept electrons to attain a full valence shell.
"Expanded octets" refers to atoms that, when bonded, must be larger than normal.
"Expanded octets" are molecules in which an element expands its valence shell to gain an octet of electrons.
"Expanded octets" is a term that describes atoms that have more than eight electrons in their valence shells.
Electrons shared between two atoms is a(n) _______.
covalent bond |
Lewis electron dot structures represent the
number of valence electrons. |
What ionic compound has the formula Na2O?
sodium oxide
Which of the following statements about the adenosine receptor is NOT true?
When adenosine is bound, a signal is sent to the cell to slow activity.
The receptor only binds adenosine.
Its normal signaling is blocked when caffeine binds.
It is involved in preparing the brain for sleep.
The receptor is found embedded in the cell membrane of neurons.
The receptor only binds adenosine.
What is the ionic formula for the ionic compound composed of calcium and chloride ions?
CaCl2
The arrow is pointing to a(n)
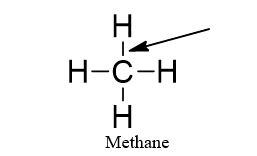
single bond. |
A compound contains magnesium and phosphate. What is the formula unit of this compound?
Mg3(PO4)2
Magnesium chloride is sometimes administered orally to suppress premature labor. Which of the following is a valid ionic formula for magnesium chloride?
None of the above choices are magnesium chloride
MgCl
Mg2Cl
Mg2Cl2
MgCl2
MgCl2
What is the name of Na2SO3, a preservative in many foods and drinks? |
sodium sulfite
Below is an illustration of an atom of fluorine. Which arrow points to the part of the atom involved in bonding?
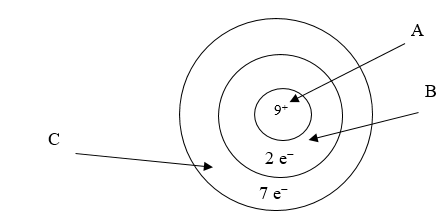
C
The name of Al3+ is ____________.
aluminum ion
Which is the BEST Lewis structure for a molecule with the formula HCN?
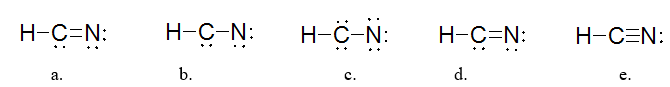
structure e |
What is the total number of valence electrons in the chloroethylene molecule (C2H3Cl)? |
18
Which of the following statements about caffeine is TRUE? |
It is consumed by the majority of North Americans.
Caffeine is a compound.
Caffeine activates the pleasure centers of the brain.
All of the above statements about caffeine are true.
Caffeine binds to the adenosine receptor.
All of the above statements about caffeine are true.
What is the name of CS2, an industrial solvent? |
carbon disulfide
Nonmetals typically ______, and metals typically ______.
accept electrons; donate electrons |
Which of the following is the formula for an ionic compound called iron(II) oxide? |
FeO
Which is the BEST Lewis structure for a molecule with the formula C2H3Cl?
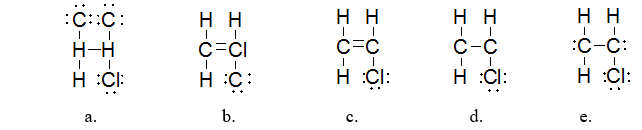
structure c
An atom of magnesium is shown below. The charge on this ion is _______ making the ion a _______.
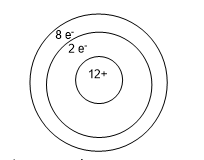
+ 2; cation
Oxygen is a _______ and therefore _______ when it forms an ion.
nonmetal; gains electrons
A high level of ______ after 12 hours of fasting indicates diabetes. |
glucose
The conventional way of writing the symbol for an ion of calcium is
Ca2+
______ is necessary for signaling in neurons and muscle cells. |
Potassium
"Laughing gas" has the formula N2O. Which of the following is the best name for this compound? |
dinitrogen monoxide
An ion that has more electrons than protons is a(n) ________. |
anion
Elements in group 1A of the periodic table are likely to form ions with the charge
+1
Which of the following ions is a polyatomic ion?
NH4+
Which of the molecules below contain an atom with an expanded octet?
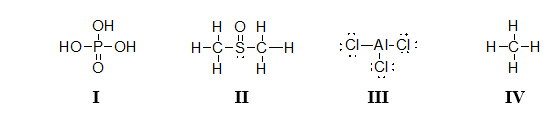
I and II
People on a low salt diet use a potassium chloride substitute for sodium chloride. Which of the following is a valid ionic formula for potassium chloride? |
KCl
How many bonds is chlorine MOST likely to form? |
1