CMS II: ENDO - EXAM #1 (ALL) (quizlet)
1/158
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
159 Terms
Basal insulin (in people without diabetes) is referred to as
low and constant levels of insulin over night and in between meals that allows the body to release sugar and other fuels from the liver
-when a healthy person hasn't eaten blood sugar levels should be between 60-100 and peak at <140 after eating
Overall effect of GLP-1, GIP, and Amylin hormones
reduce production of sugar by the liver during a meal
-diabetics tend to have sub-normal amounts of these hormones
"stress" or gluco-counter-regulatory hormones that work to increase blood sugar
Epinephrine
-promotes sugar production and breakdown and release of fat from liver
-energy for fight or flight response
Cortisol
-counterbalances insulin so puts glucose into the bloodstream
-if under stress or synthetic cortisol
(prednisone) given levels can become elevated and you can become insulin resistant
Growth Hormone
-counterbalances insulin effect on muscle and fat so high levels cause insulin resistance
T/F: type I diabetes is characterized by relative insulin deficiency
FALSE
-Type I DM is an ABSOLUTE insulin deficiency in that they have no more insulin
-in type IA, there is an autoimmune destruction of beta cells in the pancreas
-It is usually initiated by environmental factor (virus, toxic chemicals, exposure to cow's milk in infancy, cytotoxins)
-small genetic role also (HLA system)
"honeymoon phase" of type 1 diabetes refers to
stress-induced (viral illness, trauma) Epi release inhibits insulin release--> results in hyperglycemia
-this may be followed by a transient asymptomatic period known as the "honeymoon" that can last weeks to months before the onset of permanent diabetes
Amylin
beta cell hormone that is normally co-secreted with insulin in response to meals (helps to regulate glucose levels postprandially)
-it is also completely deficient in type 1 DM patients
type 1A vs type 1B diabetes
Type 1A
-type 1 diabetes that is usually in kids
-Autoimmune destruction of beta cells and HLA associated
Type 1B
-people in their 30s who develop type 1 diabetes (onset later in life)
-idiopathic and WITHOUT evidence of autoimmunity or HLA association
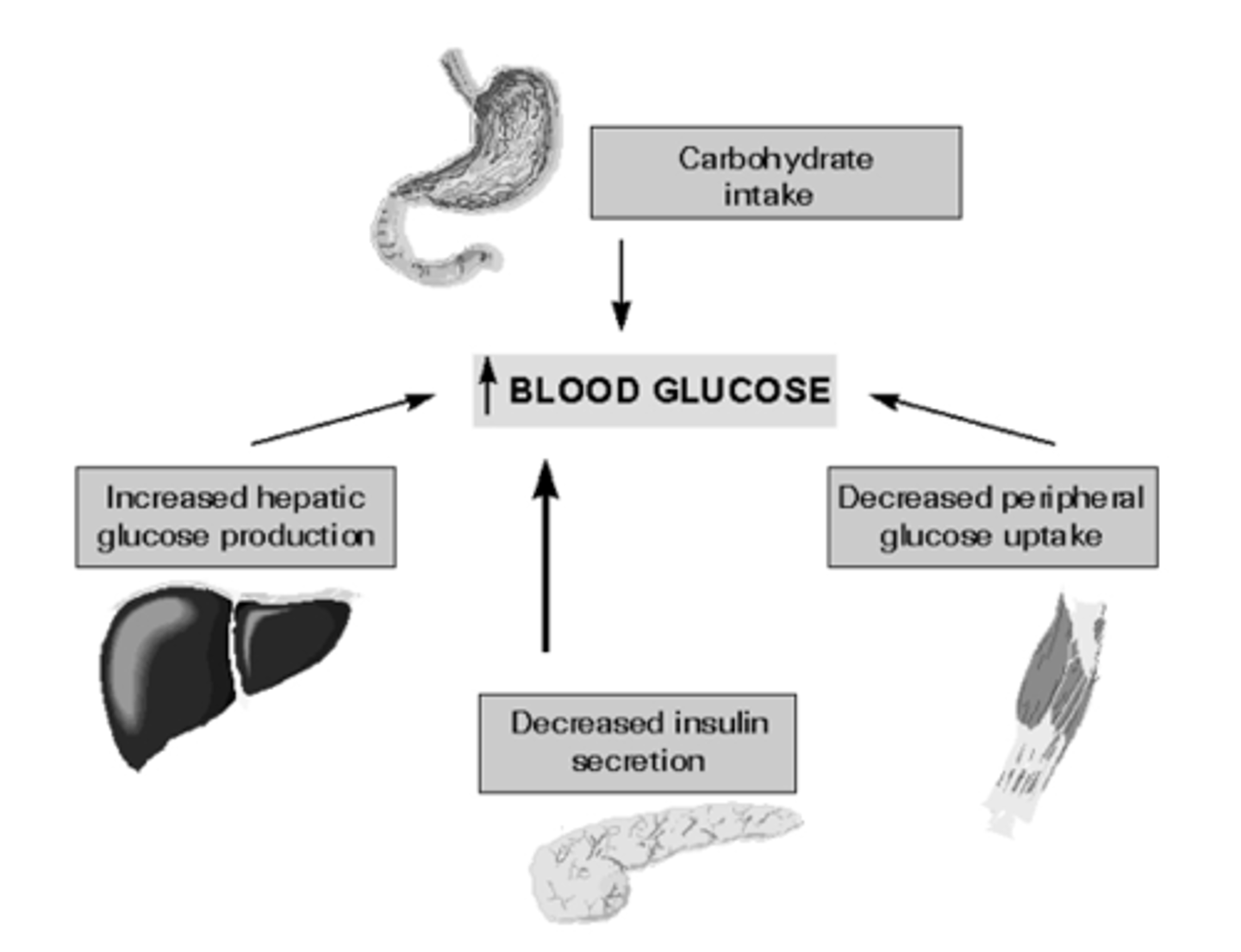
Why is type II DM referred to as relative insulin deficiency
obese patients tissues and muscles become insulin resistant so they are requiring more and more insulin to get the same effect
-beta cells eventually become over worked and then they become insulin dependent
-combo of resistance to insulins actions peripherally, inadequate insulin secretion by the pancreas, excessive glucagon secretion by alpha cellsAl
T/F: genetic input is much stronger in type II DM than type I DM
TRUE
-if anyone in the family has type II DM you have a much higher chance of also getting it
Type II DM (glucose intolerance) is part of dysmetabolic syndrome that includes
-insulin resistance
-hyperinsulinemia (need more insulin for same effect)
-obesity
-HTN
-dyslipidemia
The progression to type 2 diabetes is influenced by:
A) genetics
B) environment
C) acquired factors (sedentary lifestyle, diet that promotes obesity)
D) all of the above
D) ALL OF THE ABOVE
All overweight individuals have insulin resistance so what exactly causes diabetes to develop
those who cannot increase insulin secretion sufficiently to compensate for their insulin resistance
-for type 2 DM to occur both insulin resistance and inadequate insulin secretion must exist
-insulin concentrations are high yet inappropriately low for level of glycemia
what increases insulin requirements during pregnancy putting these patients at risk for glucose intolerance
presence of insulin antagonists (promote lipolysis and decrease glucose use):
-human placental lactogen
-chorionic somatomammotropin
-cortisol
production of insulinase by placenta also contributes to increased insulin requirements
1. Type I diabetes accounts for _____ % of diabetes cases
2. _____ people in the US have diabetes
3. Impaired glucose tolerance is present in _____ of the general population
4. Gestational diabetes develops in _____ pregnancies in the U.S.
1. 5-10%
which means type 2 diabetes accounts for 90-95% of all diabetes cases
2. 9.3% (19.3 million and nearly 6 million go undiagnosed)
3. 11%
-considered to be the most common form of glucose intolerance in the U.S.
5. 4%
highest prevalence of type 2 diabetes is found in
Pima indians of Arizona and Nauran people
Mortality/Morbidity associated with Diabetes
-7th leading cause of death in U.S. (death rate of people with diabetes is 2x that of people similar age w/o DM)
-leading cause of ESRD
-leading cause of blindness
-higher risk for heart disease, stroke, neuropathy, gangrene
Most consistent predictor of progression of diabetes in pre-diabetic patients
baseline plasma glucose
-50% of these people will progress to diabetes in 10 yrs
-pre-diabetic pts are already at inc risk of macrovascular complications
warning signs of type I DM
-polyuria (due to osmotic diuresis from hyperglycemia)
-polydipsia (from hyperosmolar state and dehydration)
-polyphagia (with weight loss)
along with fatigue, nausea, blurred vision
T/F: Type II DM is often asymptomatic so they don't realize they are diabetic until they go to the ER due to symptoms of DKA
FALSE
-Type II diabetics are often asymptomatic but they usually don't develop DKA because they have some insulin left
-Type II diabetics can present with Hyperosmolar nonketotic coma (from severe dehydration secondary to osmotic diuresis from hyperglycemia)
antecedent hx for a lot of type 2 diabetics
-frequent/recurrent infections
-poor wound healing
-blurry vision
-numbness or tingling of extremities
-may also see things similar to type I DM like polydipsia, polyphagia, polyuria
1. Since most diabetics are asymptomatic what usually triggers a screening test for glucose tolerance?
2. What is the cut off for the fasting glucose that categorizes someone as pre-diabetic?
1. presence of 1 or more cardiovascular risk factors (hx of HTN, obesity, dyslipidemia, macrovascular disease like stroke CAD PVD)
2. 126 mg/dl is the cut off

Why should you not rely just on BMI when evaluating for obese patients when it comes to type 2 diabetes?
a patient with central adiposity can have a normal BMI
-measure height, waist and hip measurements for BMI, risk level, and truncal obesity
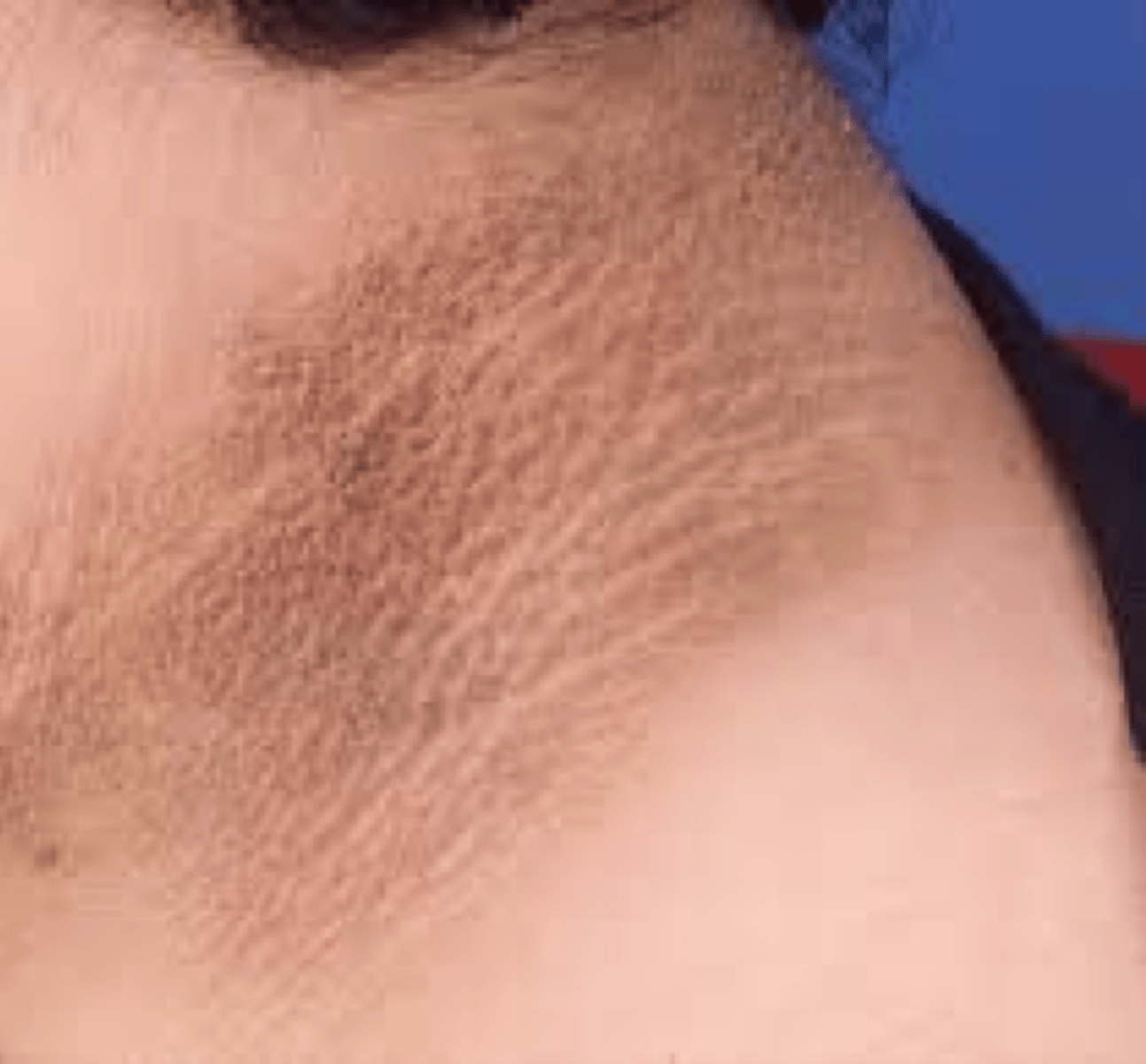
clinical features often found in type A insulin resistance patients
-Acanthosis nigricans
-features of hyperandrogenism (thin or muscular body habitus or acral enlargement)
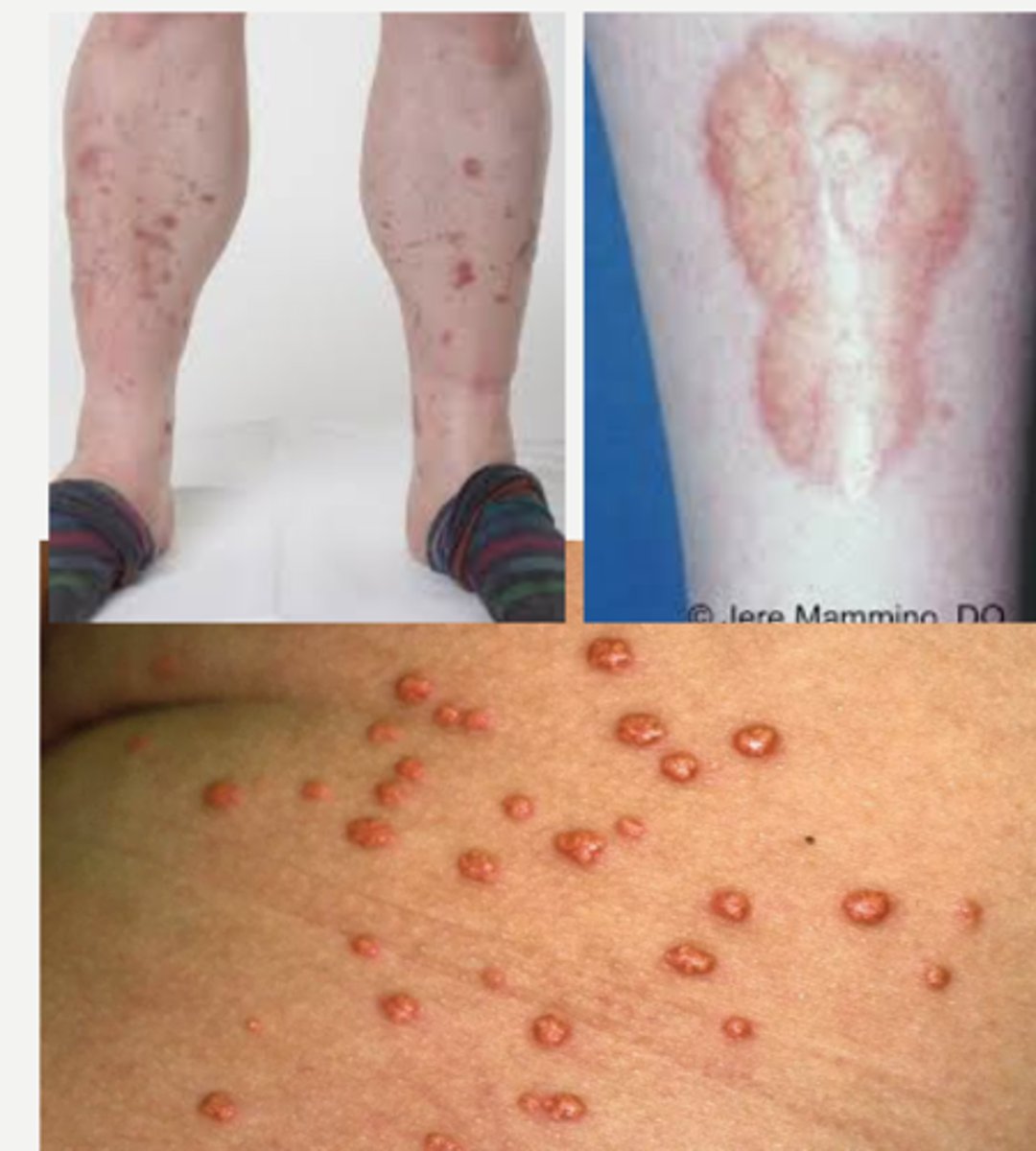
most common cutaneous finding in DM
Diabetic Dermopathy= round/oval atrophic hyperpigmented macules in pretibial areas bilaterally
other common derm findings:
-Xanthoma Eruptiva (pink papules with creamy center due to elevated TGs)
-Necrobiosis Lipodica Diabeticorum (degenerative disease of collagen characterized by atrophic waxy telangiectatic plaques or fluid filled bullae on hands and feet)
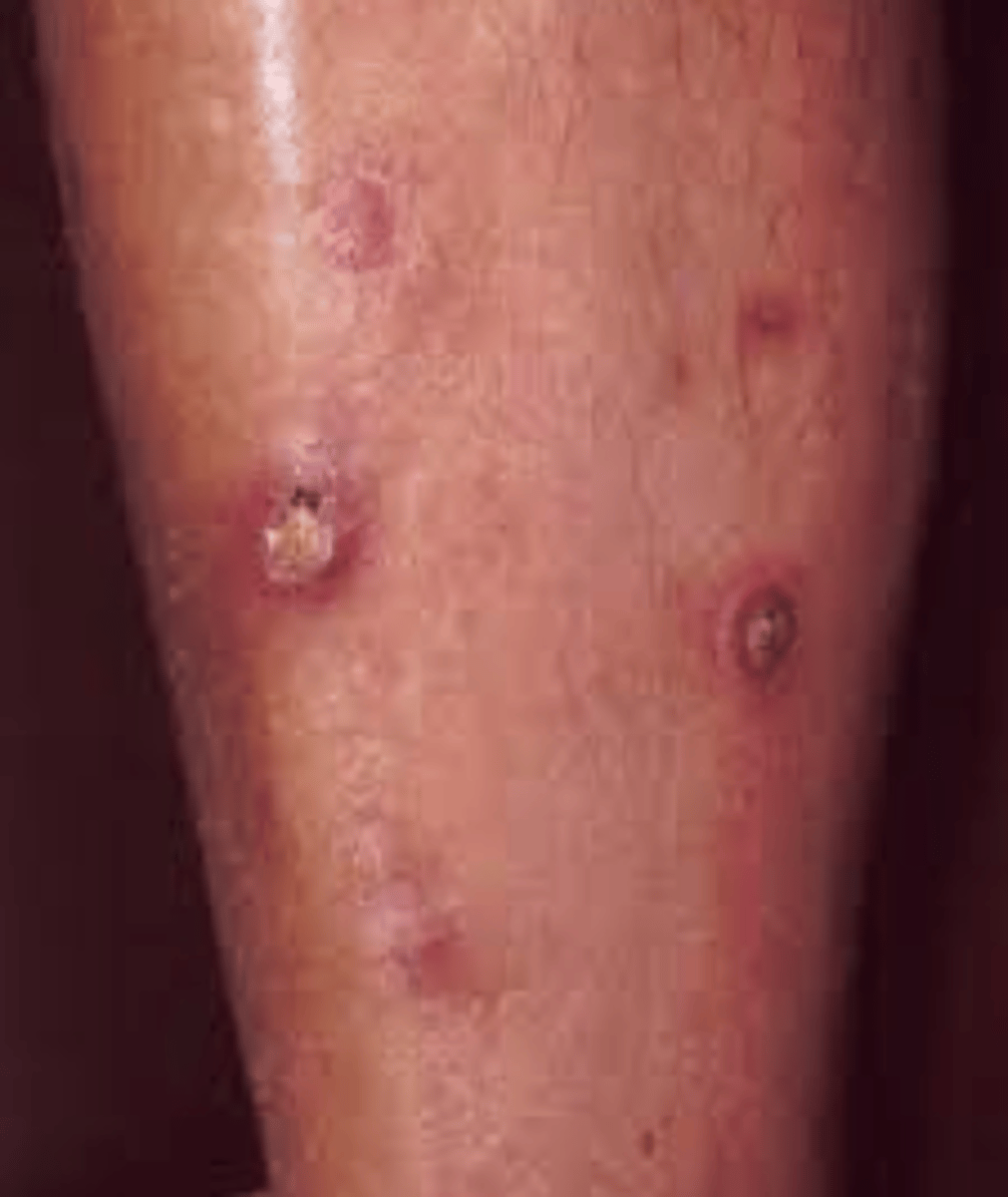
Kyrle's Disease
reacting perforating collagenosis
-puritic papules with keratotic plug on extensor surfaces with legs most commonly; associated with kidney disease
drugs with adverse effects of glucose intolerance
-thiazides
-glucocorticoids
-OCPs
-Beta adrenergic agonists
-nicotinic acid
-thyroid hormone
1. screening test for diabetes
2. preferred diagnostic test for diabetes (for someone with symptoms or abnormal screening)
1. plasma glucose measurement
2. fasting plasma glucose studies
Oral glucose tolerance test involves
measurement of plasma glucose concentration 2 hours after 75 g of oral glucose load
-this is rarely used as a confirmatory test in diagnosis of diabetes but may be helpful in situations in which fasting and random glucose results are the same
HBA1C level for pre-diabetics and diabetics
-pre-diabetic= 5.6-6.4%
-diabetic= >6.5%
note: HBA1C is highly specific as evidence of chronic hyperglycemia; predictor of chronic complications (index of severity of hyperglycemia throughout 6-8 weeks that precede the measurement)
NORMAL glucose homeostasis levels
1. HBA1C
2. Fasting plasma glucose
3. 2-hour OGTT
1. HBA1c < 5.6%
2. FPG < 100 mg/dl
3. 2 hr OGTT < 140 mg/dl after 75 g oral glucose load
PRE-DIABETES glucose levels
1. HBA1C
2. Fasting plasma glucose
3. 2-hour OGTT
1. 5.6-6.4%
2. 100-125 mg/dl
3. 140-199 mg/dl
diagnostic criteria for Diabetes Mellitus
1. HBA1C
2. Fasting plasma glucose
3. 2-hour OGTT
4. Random plasma glucose
1. 6.5% or higher
2. 126 mg/dl or higher
3. 200mg/dl or higher
4. 200 mg/dl or higher WITH classic sxs (polyuria,polyphagia,polydipsia)
screening regulation for type 2 diabetes in adults
screening tests (plasma glucose measurement) should be done at 3-year intervals in all individuals >45 yrs old
testing is indicated sooner for individuals who are BMI 25 or higher and risk factors (fam hx, HTN, inactivity)
For T2DM You should be screened before the age of 45 if you have a BMI > 25 along with what risk factors
-habitual physical inactivity
-First-degree relative w/ diabetes
-High-risk ethnic background (Hispanic, American Indian, Asian American, African American, or Pacific Islander)
-Delivery of a baby that is large for its gestational age (baby >9 lb) or history of gestational DM
-Hypertension - Blood pressure of 140/90 mm Hg or greater
-HDL cholesterol level of 35 mg/dL or less, triglyceride level of 250 mg/dL or more, or both
-Polycystic ovary syndrome (PCOS)
-Clinical conditions associated with insulin resistance (Severe obesity or acanthosis nigricans)
-History of CV disease

screening for type 2 DM in children
overweight (>85th percentile for age/sex)
plus any two risk factors:
-fam hx of T2DM in 1st or 2nd degree relatives
-high risk race
-signs of insulin resistance
-maternal hx of DM or gestational DM
screening starts at 10 yrs old or puberty and done every 3 years
screening regulations for gestational diabetes
-perform OGTT fasting at 1 hour and 2 hours
(performed in the morning after an 8 hr fast)
-performed at 24-28 weeks gestation (unless pt is high risk then do regular testing at first prenatal visit)
screen for T2DM 6-12 weeks postpartum and cont every 3 yrs
Initial tx for most patients diagnosed with pre-diabetes is lifestyle modifications which includes
-moderate weight loss (of at least 7% of total weight)
-low carb and low fat calorie restricted diet (mediterranean diet)
-Activity (minimum of 150 min per week of mod exercise)
Best tx for Gestational diabetes if lifestyle modifications fail
Insulin
-all oral agents are CI in pregnancy
T/F: if a patient's HBA1C is 6.6% pharmacologic therapy must be started immediately
FALSE
-can try lifestyle modifications first for 3 months since A1C is not greater than 7% yet
pharmacologic therapy should be considered if:
-HBA1C >7%
-Fasting glucose >126mg/dl
-Postprandial glucose level (OGTT) >160 mg/dl
Why are Biguanides (Metformin) first choice for T2DM tx (after lifestyle modifications)
-decreases hepatic gluconeogenesis (primary effect)
-increases peripheral insulin sensitivity (increases glucose uptake in peripheral tissues)
-decreases intestinal absorption of glucose
-facilitates modest weight loss
-rarely causes hypoglycemia
-CI in impaired renal function
When is Metformin CI
impaired renal function (GFR <30)
-due to risk for lactic acidosis
-max dose is 2000 mg a day and pts usually start at 500mg a day
AND
decompensated congestive heart failure
Sulfonylureas and their MOA
-Glimepiride (Amaryl)
-Glipizide (Glucotrol)
-Glyburide (DiaBeta, Glynase, Micronase)
Secretagogues that stimulate release of insulin from pancreatic beta cells
-reduce A1C by 1-2% and blood glucose by 20%
Sulfonylurea with less risk for hypoglycemia and weight gain compared to the others in its class
Glipizide (Glucotrol)
note: Glyburide is more potent and has longest half life out of them
Drugs that work as secretagogues just like Sulfonylureas
Meglitinides
-Repaglinide (Prandin)
-Nateglinide (Starlix)
stimulate insulin secretion from pancreatic cells like sulfonylureas but shorter acting and less hypoglycemia risk
Alpha-Glucosidase Inhibitors and their MOA
-Acarbose (Precose)
-Miglitol (Glyset)
MOA: slows digestion and absorption of carbohydrates in the GI tract -->slows release of carbs into the blood stream
note: these cause a lot of bowel gas
Thiazolidinediones (TZDs) and MOA
-Pioglitazone (Actos)
-Rosiglitazone (Avandia)
MOA: reduce insulin resistance in periphery--> sensitize muscle and fat to the actions of insulin
-improves target cell response to insulin without increasing insulin secretion from pancreas
most expensive oral agents
1. diabetic drug linked to increased risk of bladder cancer
2. diabetic drug with restricted access program due to increased risk for MI
1. Pioglitazone (Actos)
2. Rosiglitazone (Avandia)
GLP-1 agonists and MOA
-Exenatide (Byetta)
-Liraglutide (Victoza)
-Dulaglutide (Trulicity)
-Albiglutide (Tanzeum)
MOA: stimulates glucose-dependent insulin release, reduces glucagon, and slows gastric emptying
-injected SubQ and promotes moderate weight loss
DDP-4 inhibitors and MOA
-Alogliptin (Nesina)
-Sitagliptin (Januvia)
-Saxagliptin (Onglyza)
-Linagliptin (Tradjenta)
MOA: inactivates Dipeptidyl Peptidase-4 which is the major enzyme responsible for degrading incretin hormones-->prolongs the action of incretin hormones
Sodium-glucose cotransporter 2 inhibitors (SGLT2 inhibitors) and MOA
-Canagliflozin (Invokana)
-Dapagliflozin (Farxiga)
-Empagliflozin (Jardiance)
MOA: increases urinary glucose excretion by lowering renal glucose threshold
Amylin analogue
Pramlintide (Symlin)
-mimics endogenous amylin effects by delaying gastric emptying, decreasing postprandial glucagon release, modulates appetite
promotes satiety!! so also leads to decreased caloric intake and weight loss
Bile acid sequestrant that can be used to lower A1C
Colesevelam (WelChol)
(Mechanism unknown)
How can Bromocriptine be used to treat DM?
acts on circadian neuronal activities within hypothalamus to reset abnormally elevated hypothalamic drive for increased plasma glucose, TG, FFA levels in fasting and postprandial states in patients w/ insulin resistance
T/F: Nephropathy is a macrovascular disease that can result from uncontrolled diabetes
FALSE
-Microvascular diseases: Nephropathy, Retinopathy, Neuropathy,
Erectile dysfunction, Acute metabolic complications--> BASEMENT MEMBRANE THICKENING
-Macrovascular diseases: CAD, PVD, CVA--> ATHEROSCLEROSIS
Type I diabetics have islet cell antibodies that are directed against
Glutamic Acid Decarboxylase (GAD) within pancreatic B cells
viruses that have been hypothesized to induce an attack on beta cell function and cause Type 1 DM
-Mumps
-Rubella
-Coxsackie B4
-CMV
-Adenovirus
Most people with type II DM are obese whereas most people with Type I DM may have weight loss. Why is this?
Type I DM has polyphagia with weight loss because of the depletion of water and catabolic state with reduced glycogen, proteins, and TGs
what usually causes peripheral neuropathy in diabetics
accumulation of sorbitol in peripheral sensory nerves due to sustained hyperglycemia
If a 10 year old child who is well potty trained suddenly starts wetting the bed consistently think
Type IA DM
-nocturnal enuresis secondary to polyuria is often an indication for diabetes in children
common signs of DKA
-Kussmaul respiration
-dehydration
-hypotension
-AMS
A 12 year old female patient w/ no significant pmHx is rushed to the ER with AMS. You notice right away that she has rapid deep labored breathing. BP is 100/70 mmHg, and Na levels are 150. You also smell a fruity breath odor. What's the most likely dx? What tests do you need to order and what are you looking for to confirm Dx?
DKA
-this patient most likely has Type I DM that she was unaware of
-rapid deep labored breathing=Kussmal's respiration
tests to order:
-UA looking for ketones, glucose, protein
-Plasma acetone specifically beta-hydroxybutyrate level is HALLMARK OF DKA
-also get CBC and blood cultures to rule out infection
elevated plasma acetone beta hydroxybutyrate level think
DKA
Besides glucose tolerance testing, what other diagnostic studies can indicate type I DM?
-insulin level below 5 µU/mL
-high positive titer of glutamic acid decarboxylase antibodies suggest type 1a
-positive islet cell or insulin antibodies
Which type of diabetes usually requires higher doses of insulin?
Type II
-Type 1 diabetics are NOT insulin resistant; cells are still sensitive to insulin; they do not need very much insulin to control sugars (around 20 units a day)
-Type 2 diabetics who need insulin are already insulin resistant so much higher doses compared to type 1 pts (80-100 units a day)
How is initial insulin dose calculated
Based off weight of the patient
-then dose is divided between basal insulin and prandial insulin
when starting a pt on insulin tx, adjust the insulin dose to maintain a preprandial plasma glucose of _____
80-150 mg/dl
-insulin dose is adjusted in increments of 10% at a time and assessed over 3 days before making other changes
Calculating total daily dose of insulin for:
1. underweight, older age, or hemodialysis pts
2. normal weight pts
3. over weight pts
4. obese, insulin resistant, or pts on glucocorticoids
1. 0.3 U/kg
2. 0.4 U/kg
3. 0.5 U/kg
4. 0.6 U/kg
For a patient with a BMI of 30 who weighs 120 kg:
1. what's the total daily dose of insulin he should be taking?
2. how much insulin should he take for basal insulin?
3. how much insulin should he take for meals (if he eats 3 meals a day)?
1. For obese patients: 0.6 U of insulin/kg body weight---> 0.6U x 120 kg= 72 units insulin per day
2. 72 units/2= 36 units of insulin for basal
3. 36 units insulin/ 3 meals per day= 12 units of insulin with each meal
4 dose insulin regimen
-40-50% of total daily dose administered as long-acting at bedtime
-additional doses of rapid-acting insulin before each meal
"rule of 500" calculates
insulin:carbohydrate ratio
-500/total daily insulin dose= 1 unit of insulin to cover X grams of CHO
-from previous example total daily dose was 72 units
500/72=7
therefore 1:7 gram CHO --> 1 unit of insulin for every 7 carbs
add this amount to correction scale to account for current blood glucose
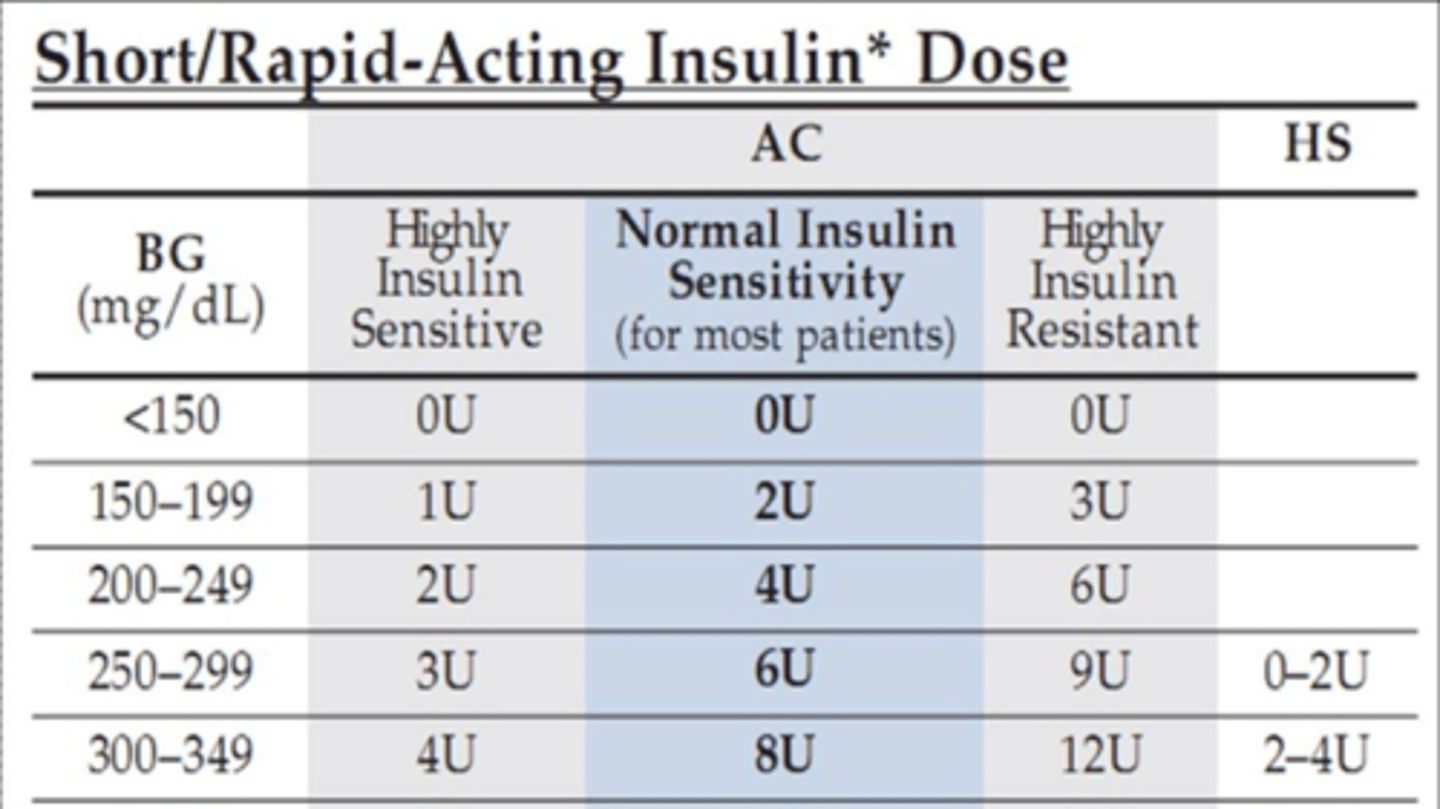
A patient with Type 1 DM checks their preprandial glucose which is 230 (sliding scale says to take 4U with this). The Patient wants to eat a 6" cold cut combo from subway which has 40g of CHO. If the patient's total daily dose of insulin is 72 units, what's the insulin carb ratio and how much insulin should they take for their bolus dosing?
insulin carb ratio= 500/72= 7
so ICR is 1:7
40g CHO x 1/7 ICR= 6 units
so needs 6 units for the meal + the 4 units to correct for current blood sugar
patient should take 10 units of insulin with lunch for bolus
what patients are appropriate to use an insulin pump
-patients whose HbA1c level is greater than 7% with frequent severe hypoglycemia (<55mg/dl)
-patients who have hypoglycemic events that require third party assistance or interfere with life activities
-patients with frequent and unpredictable fluctuations in BG
-patients who perceive that diabetes management impedes the pursuit of personal or professional goals
when should rapid and short acting insulins be used
before meals or when blood glucose is >250 mg/dl
note: long or intermediate acting insulins are better to use at night
rapid-acting (ultra-short acting) insulins (these don't "LAG")
-Humalog (insulin Lispro)
-Novolog (insulin Aspart)
-Apidra (insulin Glulisine)
onset of 5-10 min, peak at 1 hr and last 4 hrs
regular insulin is short-acting (onset is 30 min-hour and peaks at 2-5 to 5 hrs)
intermediate-acting insulins
-Neutral Protamine Hagedorn (NPH) insulin
-Lente insulin
long-acting insulins
-Ultralente insulin
-Insulin glargine (Lantus)
-Insulin detemir (Levemir)
-supply over 24 hr insulin; take it before bed to avoid hyperglycemia while sleeping
What should a diabetic patient do in regards to their insulin before exercising (rigorous exercise for at least 30 minutes)?
A) Increase their dose to avoid hyperglycemia
B)Decrease their dose to avoid hypoglycemia
C)Don't do any insulin adjustments and just eat an extra snack before working out
D)Skip a meal after working out
B)Decrease their dose to avoid hypoglycemia
OR
C) Don't do any insulin adjustments and just eat an extra snack before working out
-decrease dose by 10-20% or have an extra snack to prevent hypoglycemia during exercise--> Glut 4 receptors intake more sugar during exercise so must account for this
What is Dawn phenomenon and what could it be due to? (in type I DM)
normal tendency for blood glucose to rise in the early morning before breakfast
-may be due to nocturnal spikes in growth hormone causing insulin resistance
-probably enhanced by inc hepatic gluconeogenesis due to rise in serum cortisol
"if BG rises in the morning with the sun=Dawn phenomenon"
Somogyi phenomenon (in type I DM)
nocturnal hypoglycemia followed by marked increase in fasting plasma glucose with increase in plasma ketones
(rebound hyperglycemia from nocturnal hypoglycemia)
"BG is lOw in the middle of the night=sOmOgyi"
tx for dawn and somogyi phenomenons
administer intermediate insulin at bedtime
How often should physicians monitor well-controlled type I diabetics sugar levels and HBA1c
every 3 months
insulin resistance (type II DM) which has been attributed to free fatty acids in plasma leads to
-decreased glucose transport in muscle
-elevated hepatic glucose production
-increased breakdown of fat
T/F: increased cardiovascular risk appears to begin prior to the development of hyperglycemia
TRUE
-clock starts ticking for macrovascular risk with onset of insulin resistance AKA as soon as you are obese; before symptoms of DM even start
-clock starts ticking for microvascular risk at onset of hyperglycemia
T/F: when it comes to children, there are currently more kids being diagnosed with type I DM compared to Type II DM
FALSE
-recently there have been more type 2 than type 1 diabetes dx'ed in prepuberant children, teens, and young adults
-mainly due to rising childhood obesity
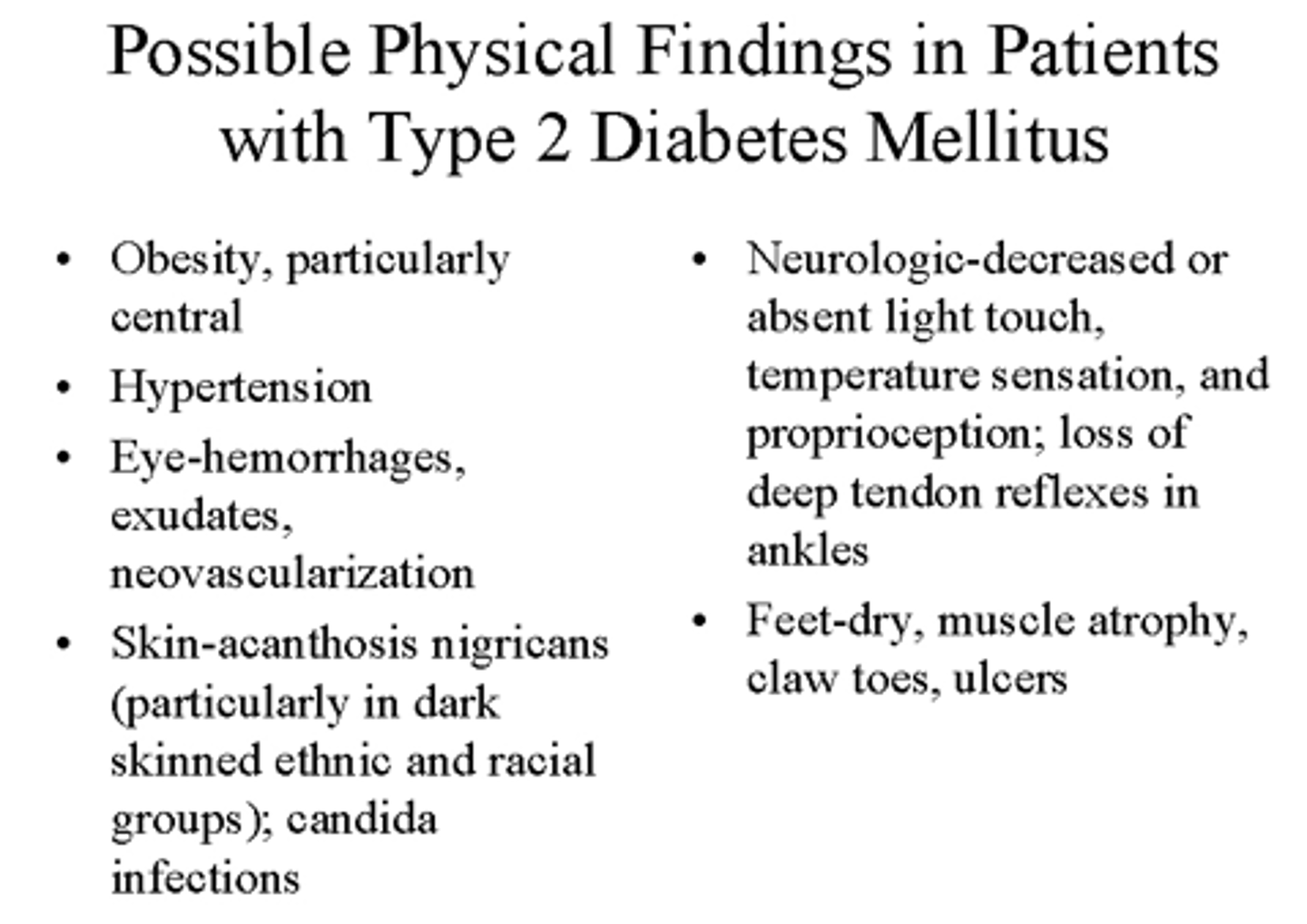
Possible physical exam findings for T2DM
other screening test done for type 2 diabetics besides glucose tolerance
microalbumin
-recommended yearly for all patients with diabetes
-if >30mg/g a timed urine specimen should be performed
-microalbuminuria is defined as 30-300 mg/d
-must confirm persistent microalbuminuria on 2 or 3 samples over 3-6 months
-if even greater values appear on dipstick screening=macroproteinuria
T/F: insulin levels are generally high early in the course of type 2 diabetes and gradually wane over time
TRUE
-pts are needing more insulin to get same effect
-over time pancreas gets overworked and blown out
remember there will be no autoantibodies in type 2 DM
__a.____ americans have diabetes and __b.___ americans have pre-diabetes
a. 30 million
b. 86 million
1. how to reduce the risk for microvascular disease
2. how to reduce the risk for macrovascular disease
1. control of glycemia and blood pressure
2. control of lipids, HTN, smoking cessation, and aspirin therapy
focus on glucose alone does not provide adequate treatment for DM pts
1. when to check A1C levels in DM patients that meet tx goals (have stable glycemic control)
2. when to check A1C levels in DM patients whose therapy has changed or who are not meeting glycemic goals
1. 2x annually (every 6 mos)
2. quarterly (every 3 months)
What can used to confirm the accuracy of a patient's meter, patient's reported self monitoring blood glucose results, or adequacy of self monitoring blood glucose testing schedule
A1C
-can also show hemolysis or blood loss
-however, A1C does not measure glycemic variability or hypoglycemia
1. reasonable A1C goal for many non-pregnant adults with DM
2. A1C goal for patients that have short duration of DM, type 2 DM treated with lifestyle modifications and metformin, long life expectancy, no significant Cardiovascular disease
3. A1C goal for patients with hx of severe hypoglycemia, limited life expectancy, advanced microvascular/macrovascular complications, extensive comorbid conditions, long-standing diabetes
1. <7%
-this has been shown to reduce microvascular complications and mortality
2. <6.5%
-only do this if it can be achieved without significant hypoglycemia or other adverse effects of tx
3. <8%
Glycemic recommendations for non-pregnant adults with DM
1. A1C
2. preprandial capillary glucose
3. peak postprandial capillary plasma glucose
1. <7.0%
2. 80-130 mg/dL
3. <180
Which of the following DM treatments requires SMBG?
A) Lifestyle modifications
B) Metformin
C) Rosiglitazone
D) Nateglinide
E) Miglitol
D) Nateglinide
-SMBG is recommended for secretagogues (Sulfonylureas and Meglitinides) and Insulin (including insulin pump) as these can cause hypoglycemic events
-SMBG not recommended for lifestyle modifcations, Biguanides, glucosidase inhibitors, TZDs because hypoglycemic risk is not as high
when should patients on multiple-dose insulin or insulin pump therapy do SMBG checks
-prior to meals and snacks
-bedtime
-prior to exercise
-when they suspect low glucose
-after treating low blood glucose until they are normogylcemic
-prior to critical tasks like driving
-sometimes postprandially
Most important aspect of diet modification for patients with DM
Caloric restriction is of first importance
-attempting to calibrate precise macronutrient composition of diet to control diabetes is generally not supported by research
In regards to medical nutrition therapy, what is recommended for patients on a fixed insulin dosing schedule?
consistent pattern of carb intake
-can improve glycemic control and reduce risk for hypoglycemia
note: if on a flexible insulin program, educate pt about carb counting
What kind of carbohydrate intake is recommended for DM patients
-whole grains
-vegetables
-fruits
-legumes
-dairy products
recommend foods higher in fiber and lower in glycemic load
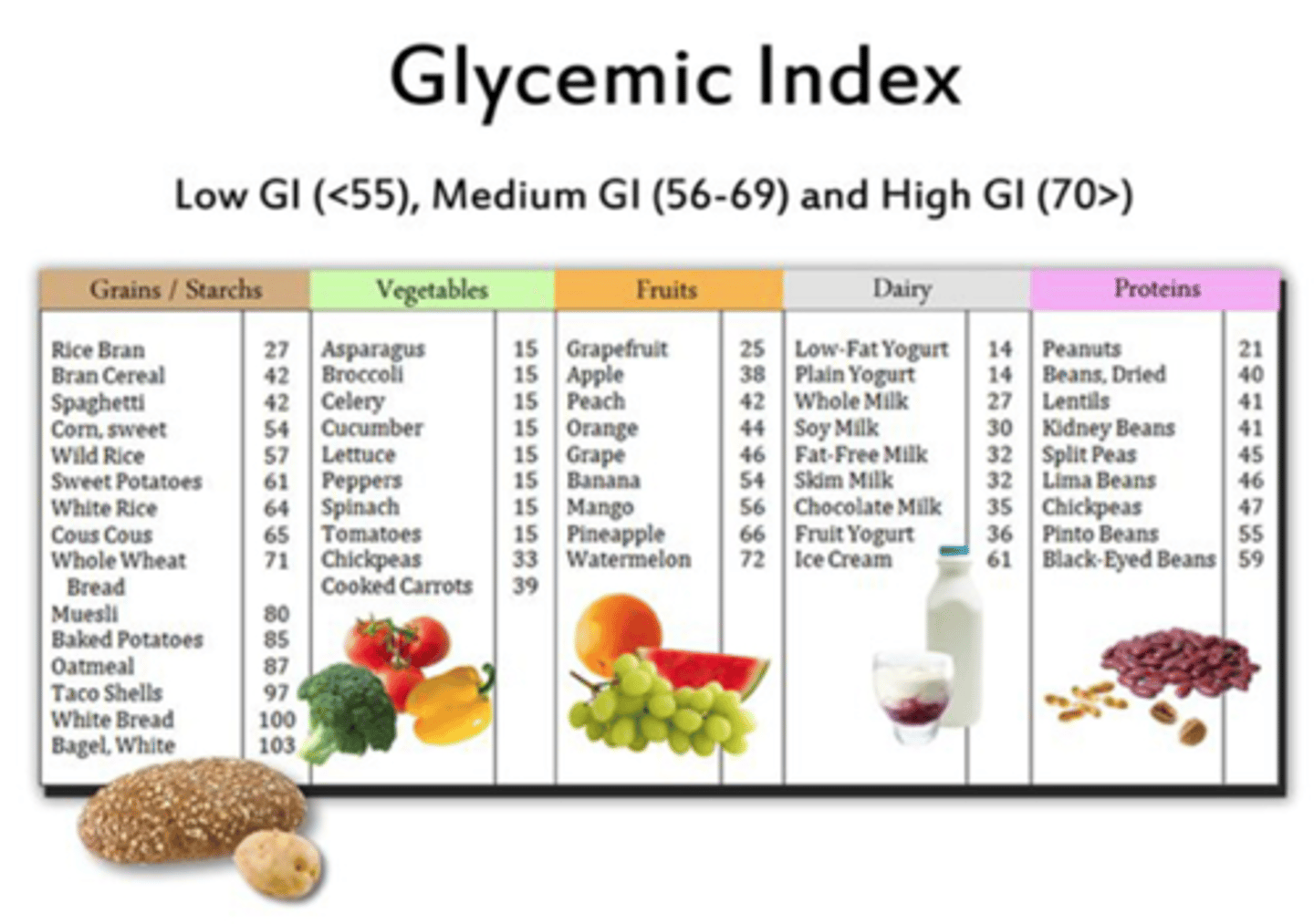
T/F: carbohydrate sources high in protein should be used to prevent hypoglycemia
FALSE
-protein increases insulin response without increasing plasma glucose concentrations
-this is good for regular meals but not for hypogylcemic events
people with diabetes should limit their sodium consumption to less than _____ a day
2300 mg/day
-further restrictions may be indicated for those with diabetes and HTN