Class 3 - Physical Thermodynamics and Fluids
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
41 Terms
What is heat? Temperature?
Heat: transfer of non-mechanical energy between a system and environment; in Joules; extensive property that depends on mass of material (size)
Temperature: macroscopic measure of internal thermal energy of a system per particle; related to KE; in Kelvin; intensive property (does not depend on amount of material)
What happens when you add heat?
Can change the phase (this does not always mean the temperature is changing)
What is the Zeroth Law of Thermodynamics?
Temperature is a fundamental property of a substance
When two substances are in contact, heat transfers between them until they reach an equilibrium temperature (thermal equilibrium)
How does heat flow?
From high temperature to low temperature
Does thermal energy acieve equilibrium?
No!
A larger object will contain more thermal energy than a small one at equal temperature
What is conduction?
Heat transfer through solids in Watts
Depends on difference in temperature and surface area of contact
No need to know equation
Slowest way to transfer heat
What is convection?
Heat transfer through fluid circulation due to warmer fluids being less dense than cooler fluids
Faster than conduction, slower than radiation
What is radiation?
Heat transfer by emission and absorption of electromagnetic energy
Fastest way to transfer heat (at speed of light)
What is the first law of thermodynamics?
Change in internal energy of a system depends on how much heat is transferred into the system and how much work the system does on its surroundings
E = energy
Q > 0 when heat is put into the system
W > 0 when the system does positive work
Doing work decreases the energy of the system
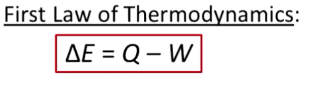
When are ideal gasses identical?
When they have the same pressure, volume, temperature, quantity (moles), and entropy → no way to identify one vs the other
They are in the same state when identical
What is the ideal gas law?
PV=nRT
P = pressure
V = volume
n = number of moles
R = gas constant
T = temperature
What are the four reversible pathways to get an ideal gas from one state to another?
Isobaric: constant pressure (delta P = 0)
Isochoric: constant volume (delta V = 0)
Isothermal: constant temperature (delta T = 0) (delta E = 0)
Adiabatic: no heat transfer (Q = 0)
What are isobaric processes? Equations?
Force = Pressure * Area
If heat transfers into the gas, the gas will expand, lifting mass by delta h and vice versa
W = F (delta H) = PA (delta H) = P (delta V)
W = work
Delta E = Q - P (delta V)

What are isochoric processes? Equations?
Volume is constant → heat transfer in will increase pressure
Delta V = 0, so W = 0
Delta E = Q
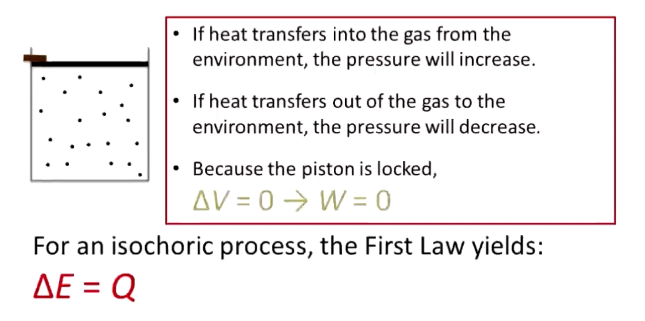
What are isothermal processes? Equations?
No temperature change → if volume increases, heat goes into the gas, and the gas expands without temperature changing
Q = W
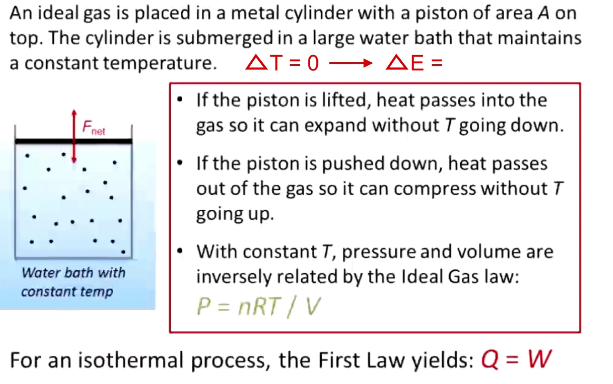
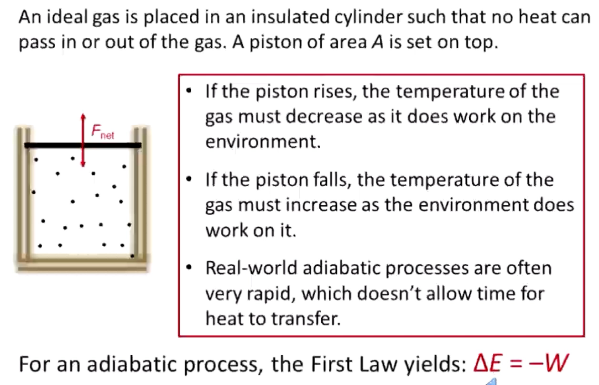
What is an adiabatic process? Equations?
No heat exchange (Q = 0)
Volume increases → temperature decreases as it does work on the environment
Often, these are things that occur too fast to allow heat transfer (ex: combustion)
Delta E = - W
What is the P-V diagram of isobaric processes?
Horizontal line at P (P constant)
Work = area under curve

What is the P-V diagram of isochoric processes?
Vertical line at V (constant V)
Work = area under curve → no work done

What is the P-V diagram of isothermic processes?
Reverse parabola
P is inversely related to V
Work = area under curve

What is the P-V diagram of adiabatic processes?
Reverse parabola below isothermic
Work = area under curve

In an ideal gas, how are internal energy and temperature related?
Directly proportional
What is a fluid?
Material that flows/takes shape of container when at rest
Liquids and gasses
What is density?
Mass/volume
In kg/m³
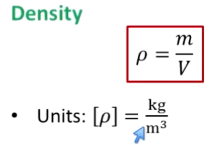
What is the weight of a fluid?
W = (density)Vg

What is specific gravity?
Ratio of density to density of water
Unitless
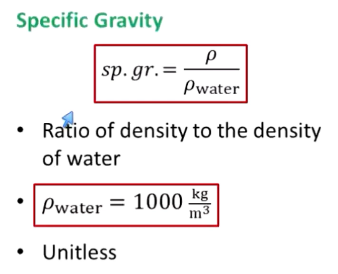
What is the density of water?
1000 kg/m³
What is pressure?
P = F/A in N/m² or Pa
F = force (exerted in all directions at once)
A = area
Scalar (force over all directions)
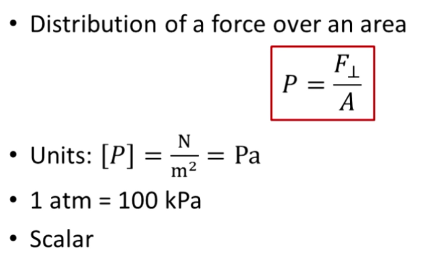
What is 1 atm in kPa?
1 atm = 100 kPa
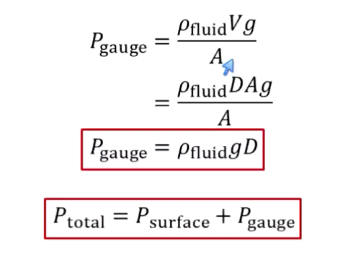
What is hydrostatic gauge pressure?
Pressure due to being immersed within fluid
Assumes gauge is zeroed at surface
P = (density of fluid)gD
D = depth
P total = P surface + P gauge
Why is there buoyant force?
Pressure is proportional to surface area and depth → proportional to force → it is greater on the bottom of the object → points up
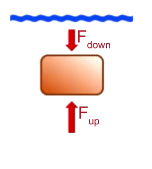
What is buoyant force?
Force exerted up on an object that is partially or completely submerged in fluid due to pressure difference between the top and bottom of the object
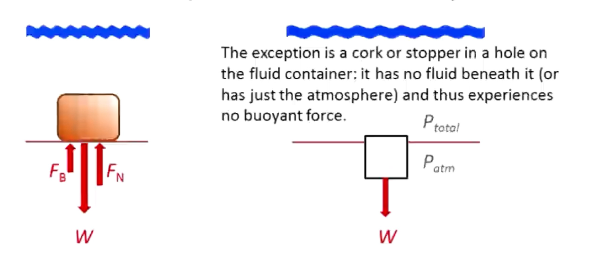
Even if you are standing on the bottom of the floor in fluid, there is buoyant force
Only a cork/stopper in a hole on a fluid container has no buoyant force
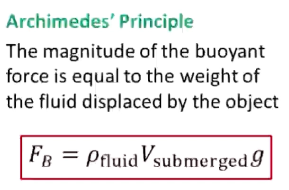
What is Archimede’s Principle?
Fbuoyant = density of fluid (Vsubmerged)g
Magnitude of buoyant force is equal to weight of fluid displaced by object
V submerged = V displaced
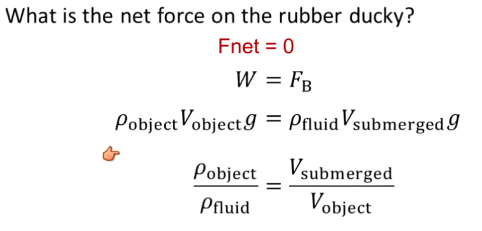
What is net force on a floating object?
Fnet = 0 = W - Fbuoyant
Density (object/fluid) = V (submerged/object)
Gravity will not affect floating
Cannot displace more than your volume
Must be less dense than the fluid to float
What is the buoyant force on two objects floating in the same substance?
The same
Equal
What is flow rate?
Volume of a fluid moving through a particular cross-sectional area per unit time
f = m³/s
If constant across an area (ex: pipe): f = Av, where A is cross sectional area and v is velocity
What is continuity?
For an incompressible (constant density) fluid, flow rate is constant through a pipe
Reducing area → increasing speed
A1v1 = A2v2
What is an ideal fluid?
Incompressible (constant density)
Negligible viscosity (no intrafluidic friction)
Laminar (streamline) flow: no turbulence, eddies, or crossing streams
Flow rate is steady
What are important fluids that are not ideal?
Blood → it is viscous
Air in lungs can become turbulent in certain illnesses
What is Bernoulli’s equation?
Describes ideal fluids
Conservation of energy for fluids
P1 +(density)gy1 + 1/2(density)v1² = P2 + (density)gy2 + 1/2(density)v2²
P = pressure
v = velocity
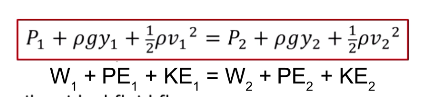
What are two special cases to Bernoulli’s law?
At any two points of equal height, faster fluid flow means lower pressure
Any fluid exposed to the atmosphere is at atmospheric pressure
What is the efflux velocity of water coming out of a hole in an exposed top bucket?
Vefflux = sqrt(2gD)
D = depth to top of hole