AP Chemistry Unit 2
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
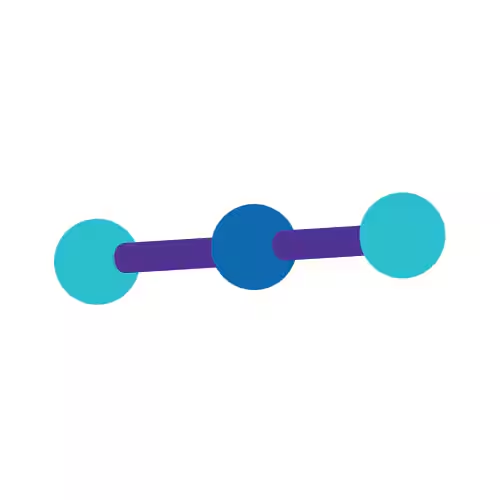
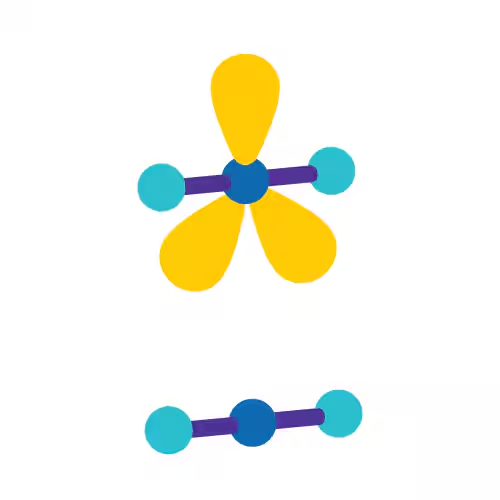
Linear
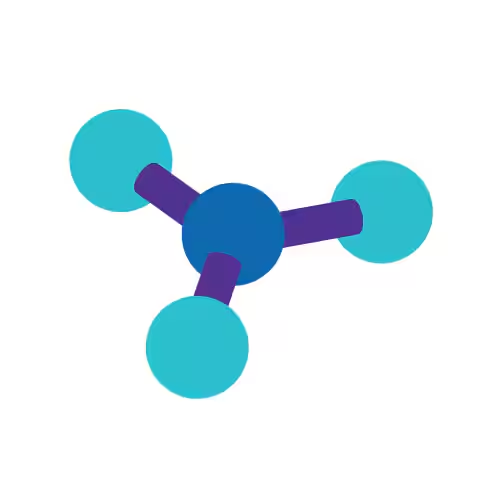
Trigonal Planar
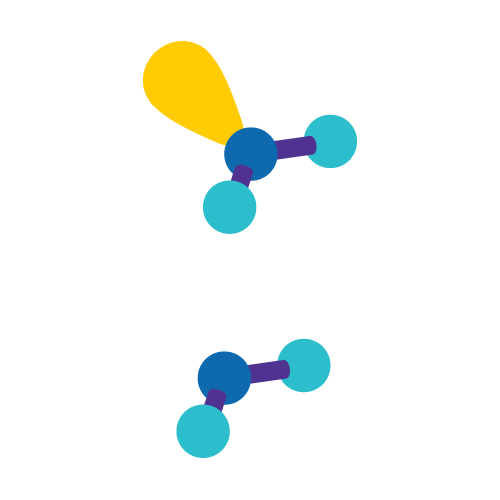
Angular/Bent
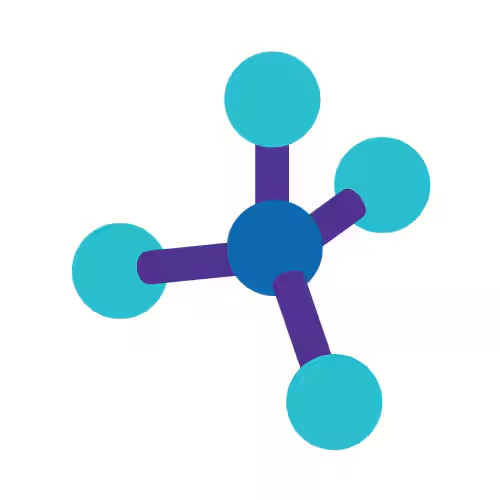
Tetrahedral
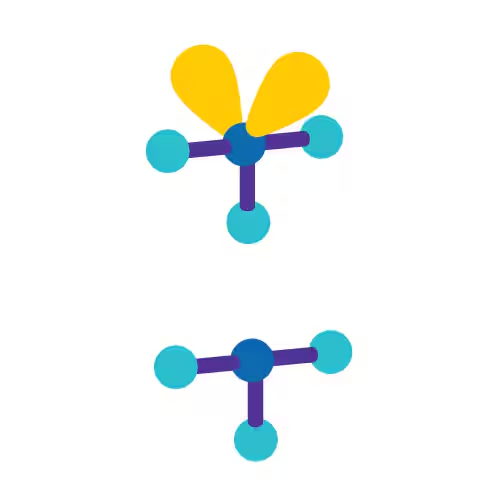
Trigonal Pyramidal
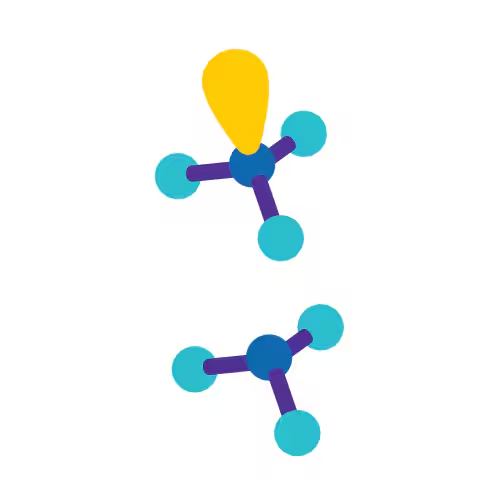
Angular/Bent
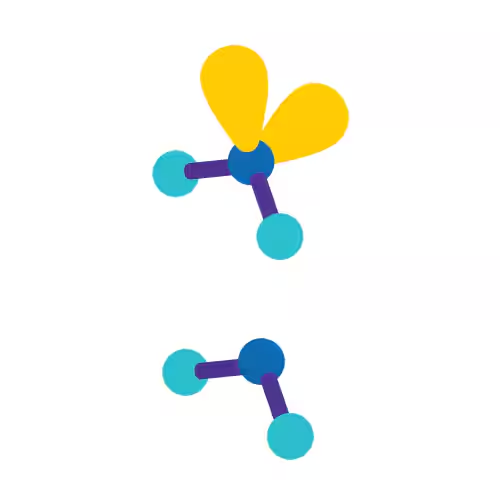
Trigonal Bipyramidal
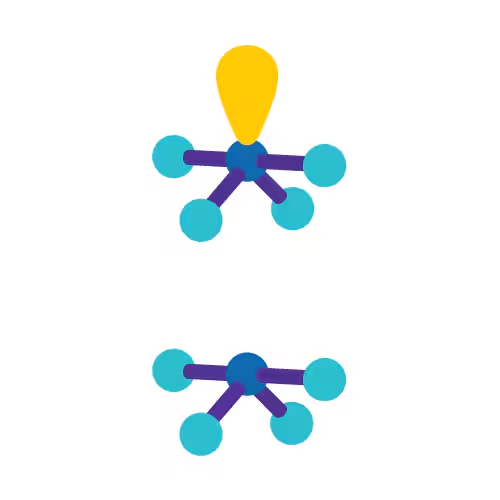
Seesaw
T-Shaped
Linear
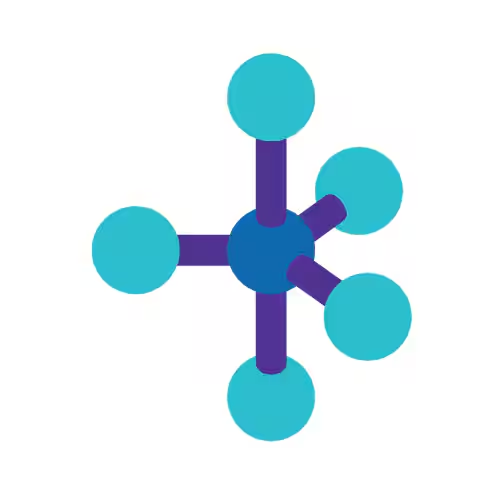
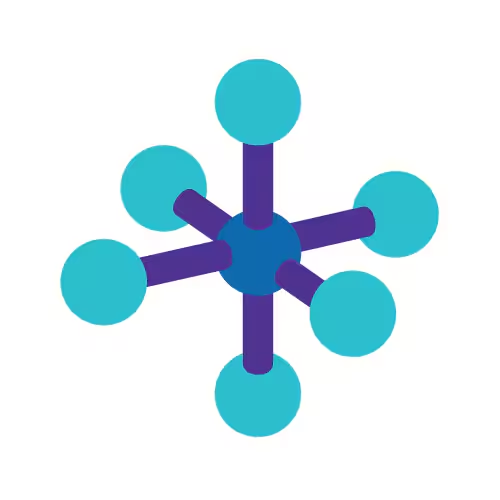
Octahedral
Square Pyramidal
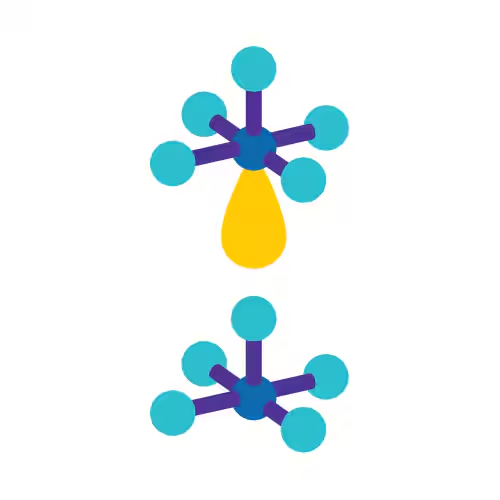
Square Planar
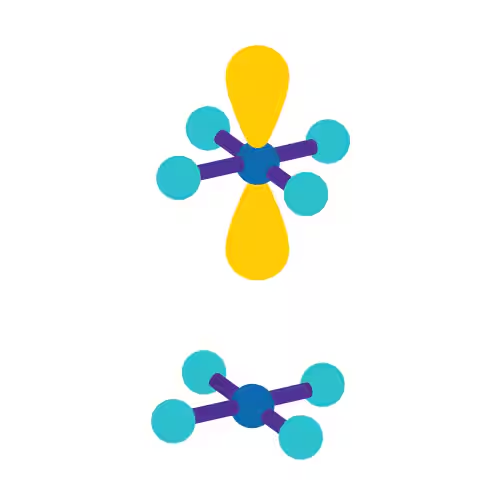
sp
Linear
sp2
Trigonal Planar
sp3
Tetrahedral
sp3d
Trigonal Bipyramidal
Single bond
Longest and weakest bond
Triple bond
Shortest and strongest bond
LDF
Weakest intermolecular force occurring in every molecule from temporary dipoles.
Dipole-dipole
Intermolecular force occuring between the oppositely charged ends of two polar molecules.
Hydrogen Bond
Intermolecular force occurring between Hydrogen and Nitrogen, Oxygen, or Fluorine.
Ion-dipole force
Force of attraction between an ion and a polar molecule. Usually found when ionic compounds are dissolved in polar solvents
Right
Which way does the dipole moment point?
σ+ ___ σ-
Left
Which way does the dipole moment point?
σ- ___ σ+
Interstitial Alloy
Alloy formed between atoms with large differences in radii.
Substitutional Alloy
Alloy formed between atoms with similar radii.
Resonance Structure
Set of 2 or more lewis structure that collective describe the electronic bonding of a single polyatomic species.
3
Minimum period required for an expanded octet.
VE - UE - BE/2
Formal Charge Formula
Sigma bond
“Head-to head” atomic orbital overlap; strongest covalent bond.
Pi bond
Lateral atomic orbital overlap; prevents rotation in covalent bonding.