Chapter 6 Anatomy
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
49 Terms
Flat bone
Bone that has a wide surface
Short bones
Bones of the wrist
Long bones
Bones of the thigh
Irregular bones
Vertebrae are examples of these
Flat bones
The bones of the skull that form a protective covering for the brain are examples of these
Long bone
A bone that provides a rigid rod (lever) for skeletal muscle to pull upon during contraction
Special type of short bone called sesamoid bone
Patella (kneecap) is an example of this
Why doesn’t the periosteum cover the articular cartilage of a long bone?
So that there is no bone on bone contact.
Location of periosteum
Around bone (except articular cartilage)
Tissue of periosteum
Dense irregular connective tissue
Location of endosteum
Lines medullary cavity
Tissue of endosteum
Loose reticular tissue
Laura is in her mid-50s and has recently been diagnosed with osteopenia, a term used for a lower- than-normal bone mass. Her physician told her that this diagnosis is “an opportunity.” What do you think the physician means?
That this is an opportunity is to take better care of her bone health.
Describe the experiment Gabel and her colleagues set up
They used high-resolution peripheral quantitative computed tomography (HR-pQCT) to image the bone structure of tibia in the lower leg and the radius in the lower arm.
Gabel and colleagues’ experiment results
Astronauts in space for less than six month lost bone strength but were able to gain it back in a year after being in Earth’s gravity. But those in space for longer had permanent loss in their shinbones, or tibias, equivalent to a decade of aging.
Gabel and colleagues’ results measurement
The images were taken at 4 points in time: before spaceflight, when astronauts returned from space, six months and one year later.
Why did radii lose less bone mass than tibias after at least six months in space?
The radii aren’t weight-bearing bones.
What type of bone can never be rebuilt after it is gone?
Trabeculae
What type of measures are being considered to mitigate bone loss on longer spaceflights?
More nutrition (such as calcium and vitamin D) and exercise (because it stimulates osteoblasts (which are bone-building cells))
Comminuted fracture
Bone breaks into many fragments (particularly common in the aged, whose bones are more brittle).
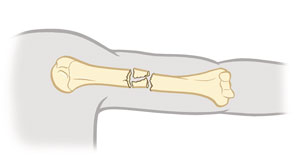
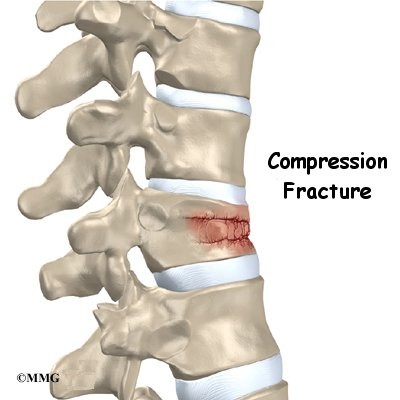
Compression fracture
Bone is crushed i.e., osteoporotic bones (common in porous bones)
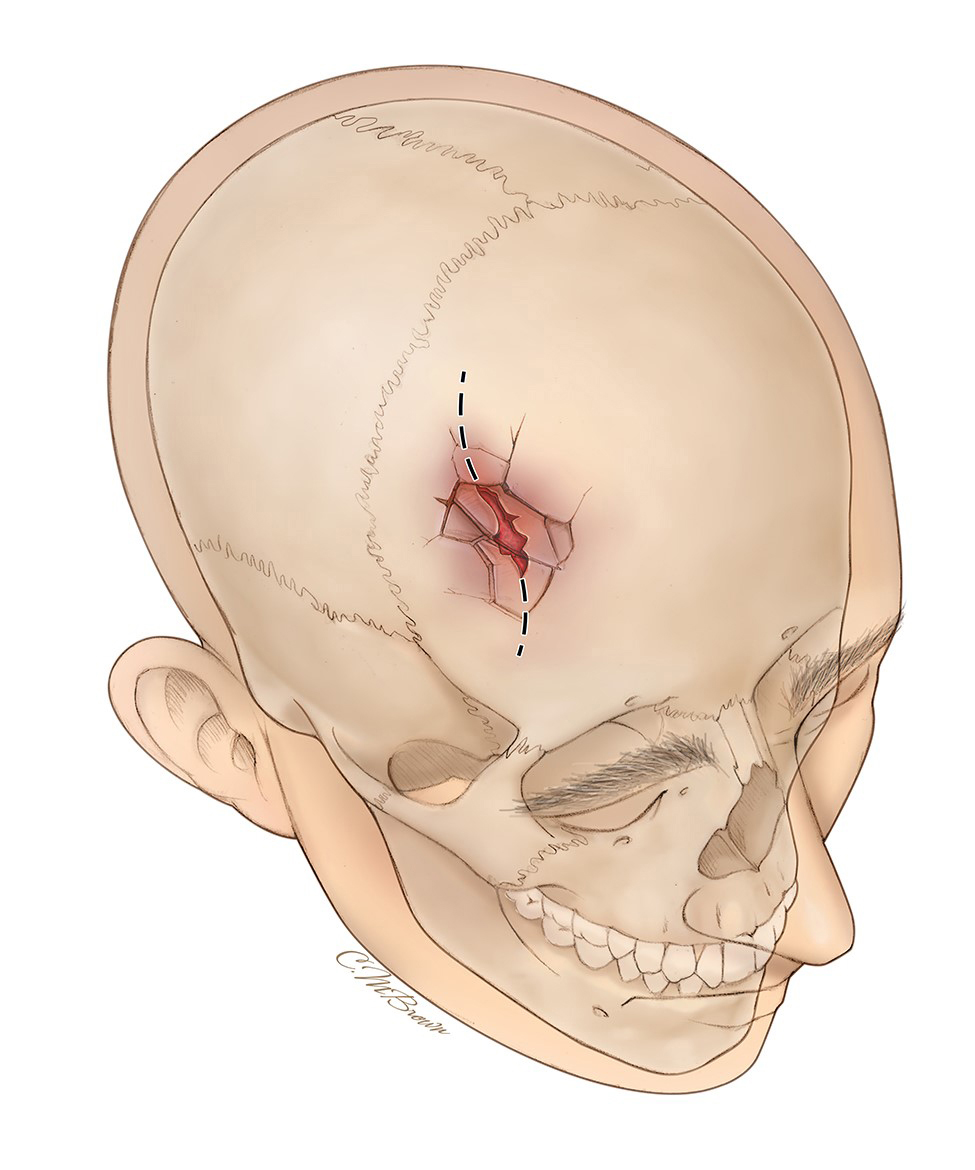
Depressed fracture
Broken bone portion is pressed inward
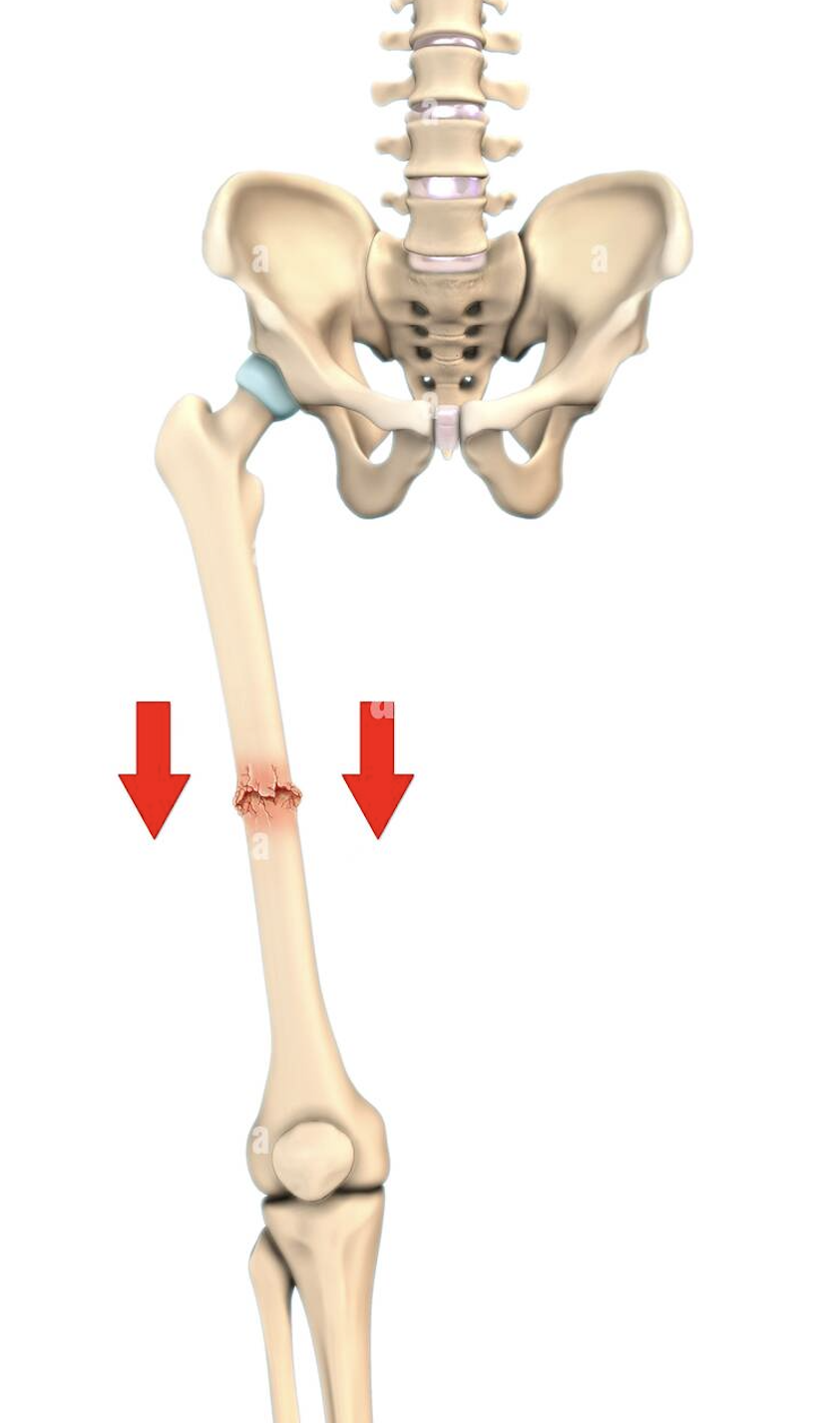
Impacted fracture
Broken bone ends are forced into each other (commonly occurs when one attempts to break a fall with outstretched arms)
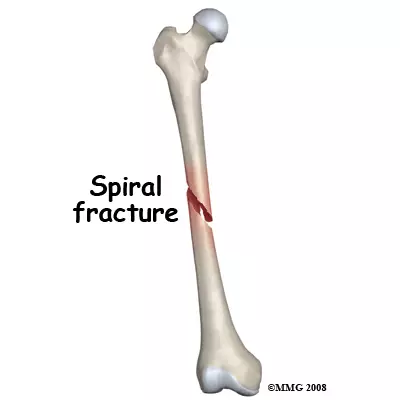
Spiral fracture
Ragged break occurs when excessive twisting forces are applied to a bone (common sports fracture)
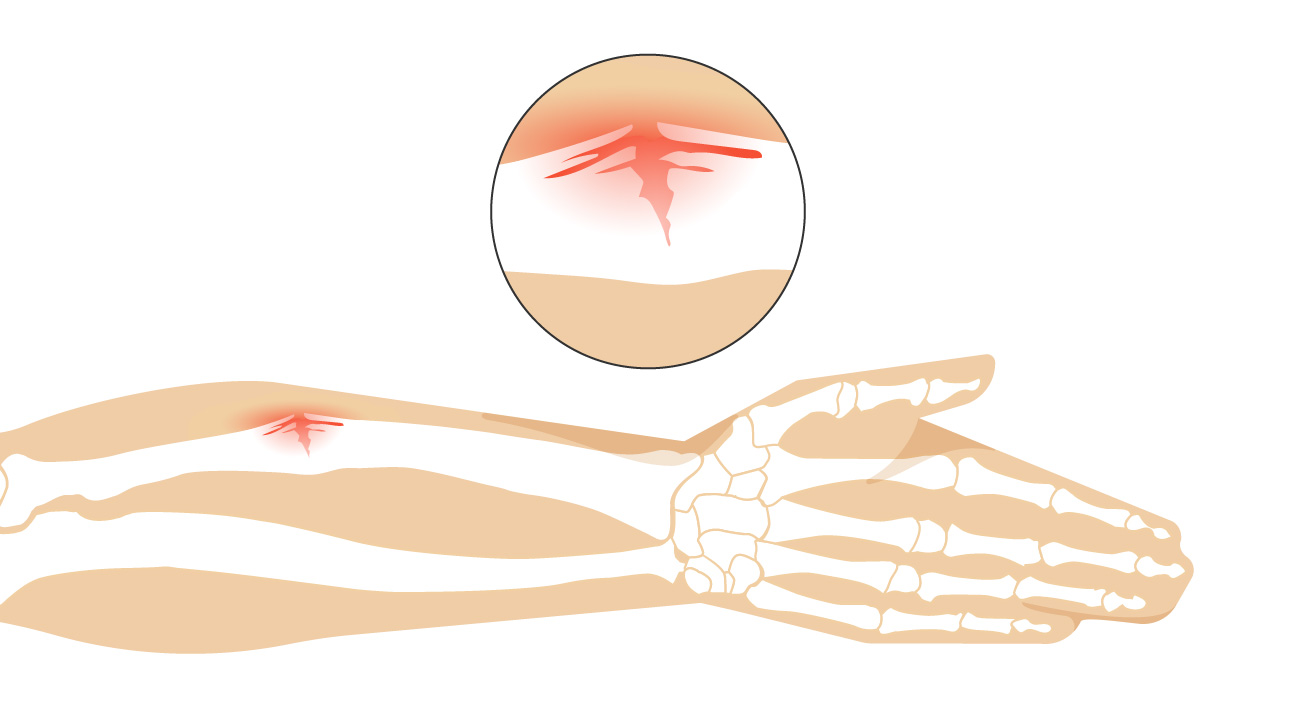
Greenstick fracture
Bone breaks incompletely (common in children, whose bones are more flexible than those of adults)
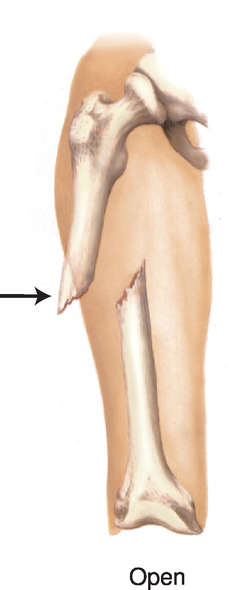
Open fracture
The broken ends of the bone protrude through the skin.
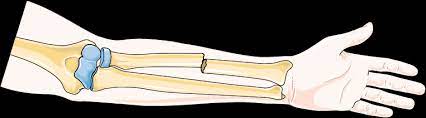
Closed fracture
It does not break the skin.
1 stage of bone remodeling after a fracture occurs
Reactive phase
2a stage of bone remodeling after a fracture occurs
Reparative phase: Fibrous cartilage callus formation
2b stage of bone remodeling after a fracture occurs
Reparative phase: Bony callus formation
3 stage of bone remodeling after a fracture occurs
Bone remodeling phase
Differences between intramembranous and endochrondal ossification
Intramembranous is simpler, forms most bones of body, forms directly within mesenchyme. Endochrondal is more complex, forms flat bones of skull, most facial bones, mandible (lower jawbone) and medial part of clavicle, forms within hyaline cartilage that develops from mesenchyme.
Similarities between intramembranous and endochrondal ossification
Both are ways to form bones.
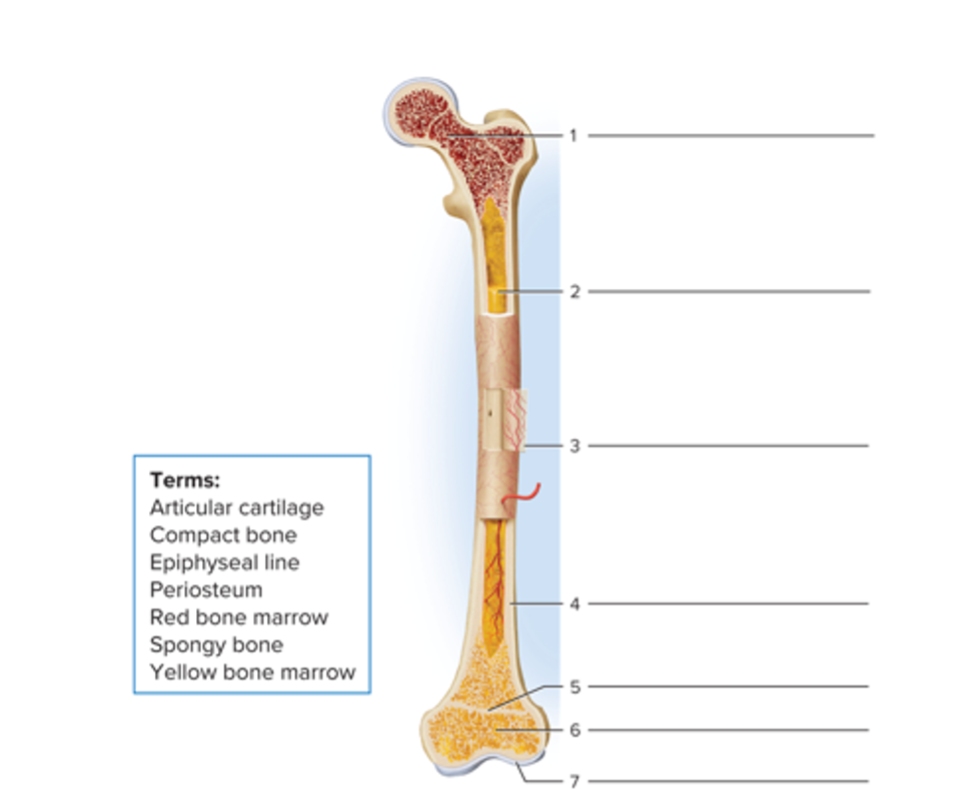
Parts of a long bone
Red bone marrow
Yellow bone marrow
Periosteum
Compact bone
Epiphyseal line
Spongy bone
Articular cartilage
Zones in epiphysis of a lengthening bone
Resting cartilage, proliferating cartilage, hypertrophic cartilage, calcified cartilage
Zone of resting cartilage
This layer is nearest the epiphysis and consists of small, scattered chondrocytes. The term “resting” is used because the cells do not function in bone growth in length. Rather, they anchor the epiphyseal plate to the epiphysis of the bone.
Zone of proliferating cartilage
Slightly larger chondrocytes in this zone are arranged like stacks of coins. These chondrocytes undergo interstitial growth as they divide and secrete extracellular matrix. The chondrocytes in this zone divide to replace those that die at the diaphyseal side of the epiphyseal plate.
Zone of hypertrophic cartilage
This layer consists of large, maturing chondrocytes arranged in columns.
Zone of calcified cartilage
The final zone of the epiphyseal plate is only a few cells thick and consists mostly of chondrocytes that are dead because the extracellular matrix around them has calcified. Osteoclasts dissolve the calcified cartilage, and osteoblasts and capillaries from the diaphysis invade the area. The osteoblasts lay down bone extracellular matrix, replacing the calcified cartilage by the process of endochondral ossification. Recall that endochondral ossification is the replacement of cartilage with bone. As a result, the zone of calcified cartilage becomes the “new diaphysis” that is firmly cemented to the rest of the diaphysis of the bone.
Effects of exercise on bone
Helps to build and retain bone mass. Bone tissue becomes stronger through increased deposition of mineral salts and production of collagen fibers by osteoblasts.
Effects of aging on bone
Loss of bone mass due to demineralization and brittleness due to lack of collagen fibers.
Effects of reducing estrogen and testosterone levels
Reduces osteoblast activity and the synthesis of bone matrix
Calcium homeostasis hormones
Calcitonin (CT), parathyroid hormone (PTH), Calcitriol
Calcitonin function
Inhibits activity of osteoclasts, speeds blood Ca2+ uptake by bone, and accelerates Ca2+ deposition into bones. The role of CT in normal calcium homeostasis is uncertain because it can be completely absent without causing symptoms.
Parathyroid hormone function
PTH secretion operates via a negative feedback system. If some stimulus causes the blood Ca2+ level to decrease, parathyroid gland cells (receptors) detect this change and increase their production of a molecule known as cyclic adenosine monophosphate (cyclic AMP). The gene for PTH within the nucleus of a parathyroid gland cell (the control center) detects the intracellular increase in cyclic AMP (the input). As a result, PTH synthesis speeds up, and more PTH (the output) is released into the blood. The presence of higher levels of PTH increases the number and activity of osteoclasts (effectors), which step up the pace of bone resorption. The resulting release of Ca2+ from bone into blood returns the blood Ca2+ level to normal.
Calcitriol function
A hormone that promotes absorption of calcium from foods in the gastrointestinal tract into the blood. Both of these actions also help elevate blood Ca2+ level. If some stimulus causes the blood Ca2+ level to decrease, the thyroid gland releases calcitonin which stimulates Ca2+ deposition in bones (osteoblasts increase) and reduces Ca2+ uptake in kidneys. The result is that blood Ca2+ declines to set point.
Calcitonin (CT) blood calcium
Lowers blood calcium
Parathyroid hormone (PTH) blood calcium
Raises blood calcium
Calcitriol
Raises blood calcium