Lecture 8: Immunity Mediated by B Cells and Antibodies with 100% accurate questions and answers 2025-2026
1/88
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
89 Terms
what is the finding and implication of "Marginal zone B cells acquire dendritic cell functions by trogocytosis"
Finding: The study highlights a novel mechanism by which marginal zone (MZ) B cells acquire major histocompatibility complex class II (MHC II) molecules from conventional dendritic cells (cDCs) through a process called trogocytosis. Specifically, the study describes how MZ B cells use complement receptor 2 (CR2) to recognize and capture MHC II molecules that are bound to complement component 3 (C3) on cDCs. This interaction allows MZ B cells to display the MHC II–C3 complexes on their own surfaces, effectively adopting cDC-like properties for antigen presentation.
Implications: This finding provides significant insights into the adaptive capabilities of MZ B cells, particularly in how they participate in the immune response beyond their traditional role in antibody production. By acquiring and presenting MHC II–C3 complexes, MZ B cells can engage in antigen presentation to T cells, a key function typically associated with dendritic cells. This ability enhances the immune system's flexibility and responsiveness, especially in early life when robust immune protection is critical.
what are the 3 phases by which B cells become activated via cross-linking of surface immunoglobulin
1. cross linking of B cells by ag
2. clustering of ag receptors allows receptor associated kinases to phosphorylate the ITAMs
3. Syk binds to doubly phosphorylated ITAMs and is activated on binding this then leads to activating signals and changes in gene expression in nucleus
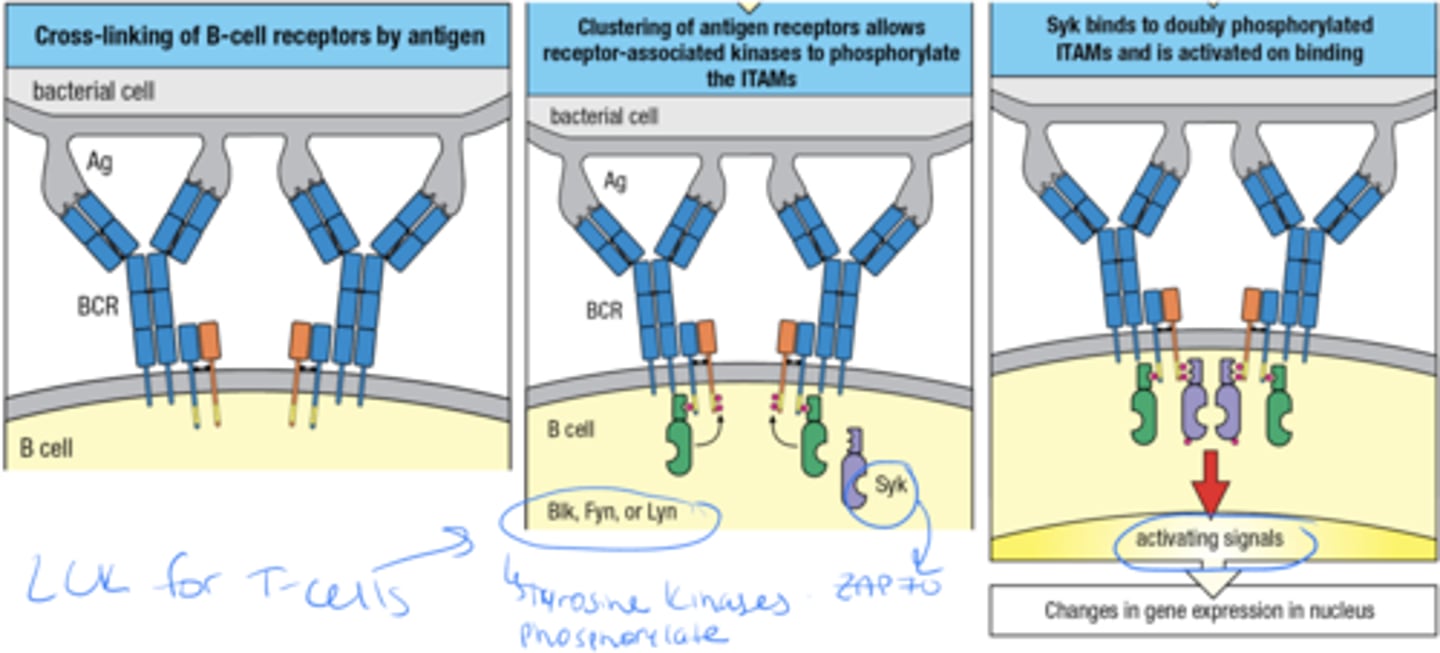
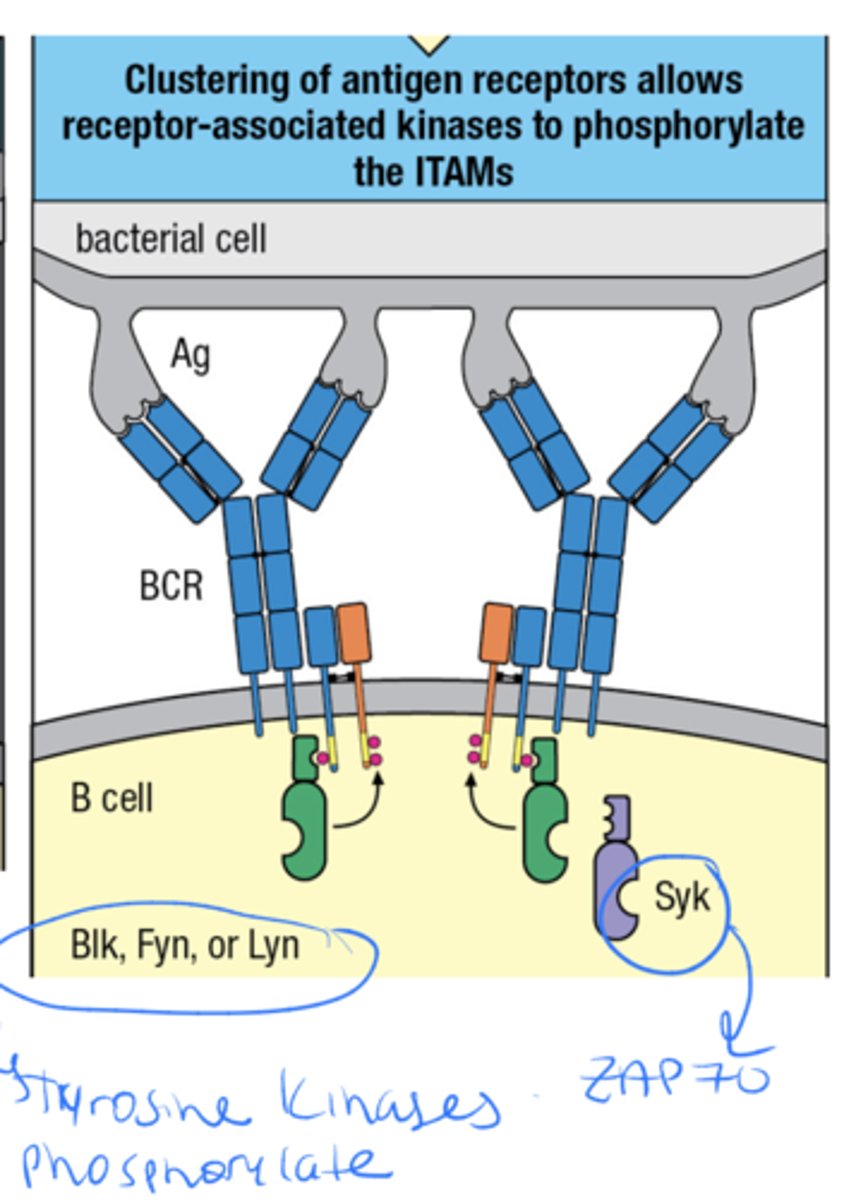
what are the ITAMs, where are they located and what 3 things interact with them
ITAMs are are sequences on the cytoplasmic tails of certain immune receptors and the 3 things that interact with them are Src-family Kinases such as Blk, Fyn, Lyn
On binding to specific epitopes, the surface IgM molecules of naïve, mature B cells become
physically cross-linked to each other
This clustering and aggregation of BCRs sends signals
inside the cell through Iga and Igb
B-cell activation requires signals from the
B-cell co- receptor
The B cell co receptor is made up of what 3 parts
CR2
CD19
CD81
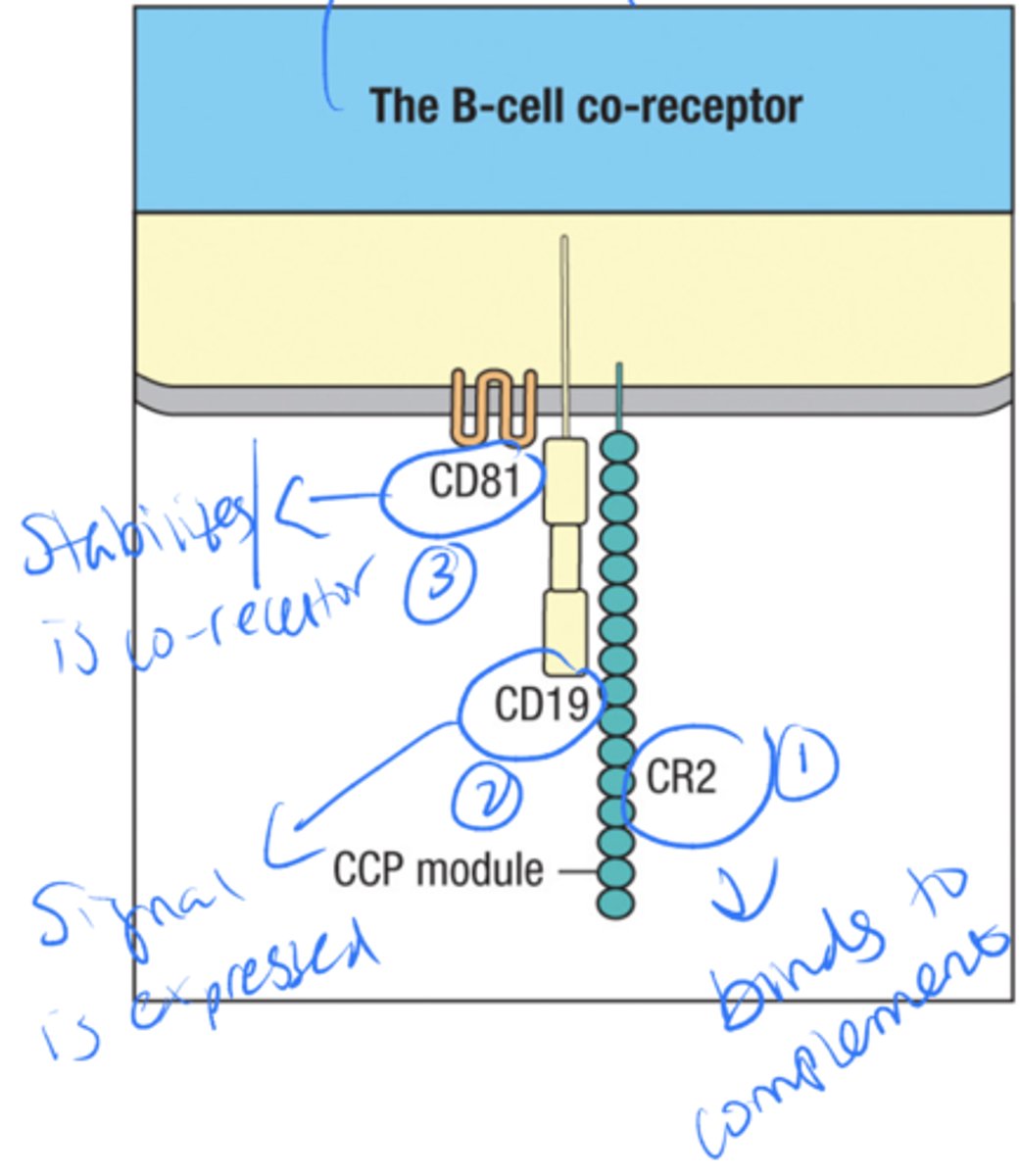
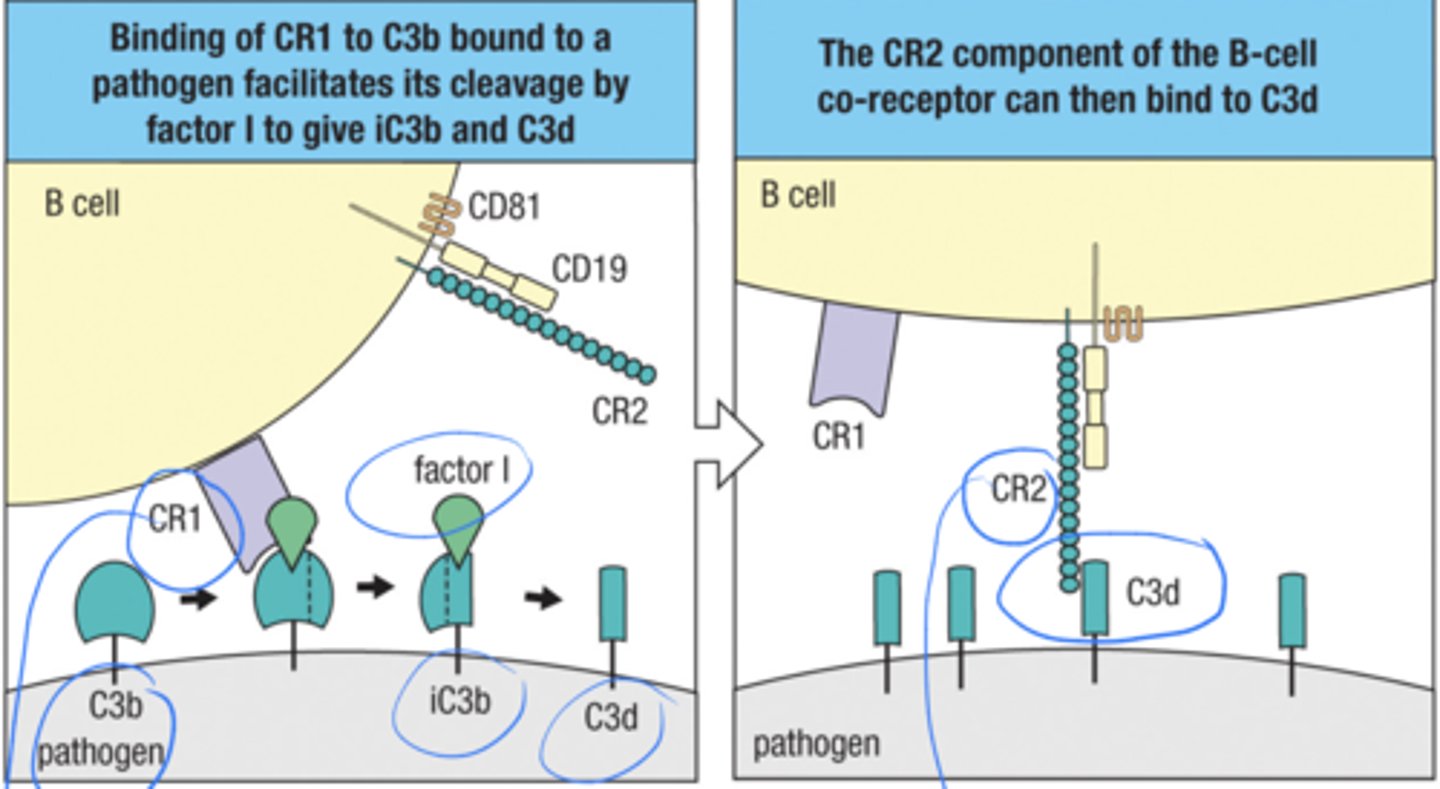
what is the signaling for the B cell co-receptor
1. binding of CR1 to C3b bound to a pathogen facilitates its cleavage by factor I to give iC3b and C3d
2. The CR 2 component of the B-cell co receptor can then bind to C3d
B-cell activation requires signals from the B-cell co- receptor what is the 2 step process
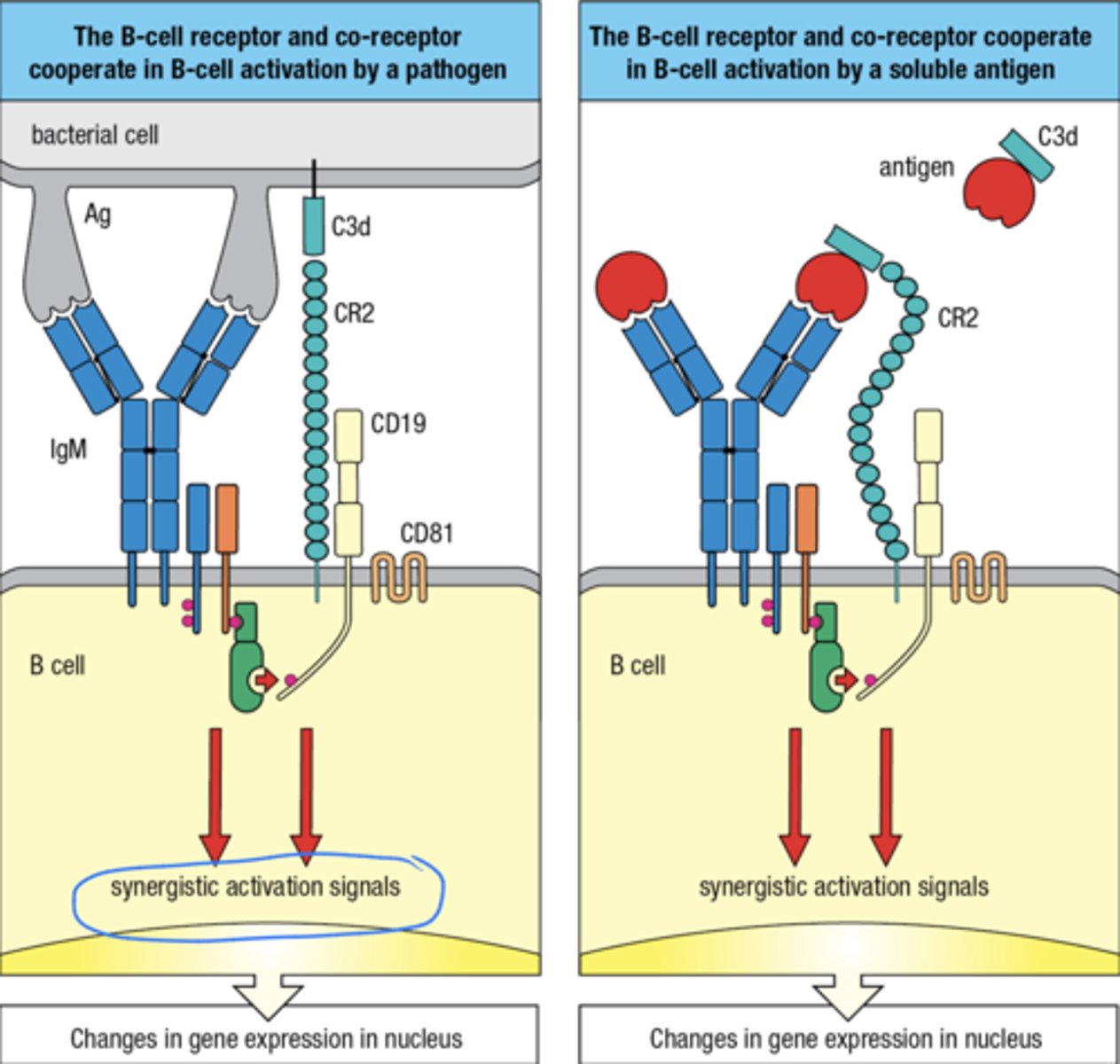
1. The B cell receptor and the co-receptor cooperate in B cell activation by a pathogen
2. The B cell receptor and co-receptor cooperate in B cell activation by a soluble ag
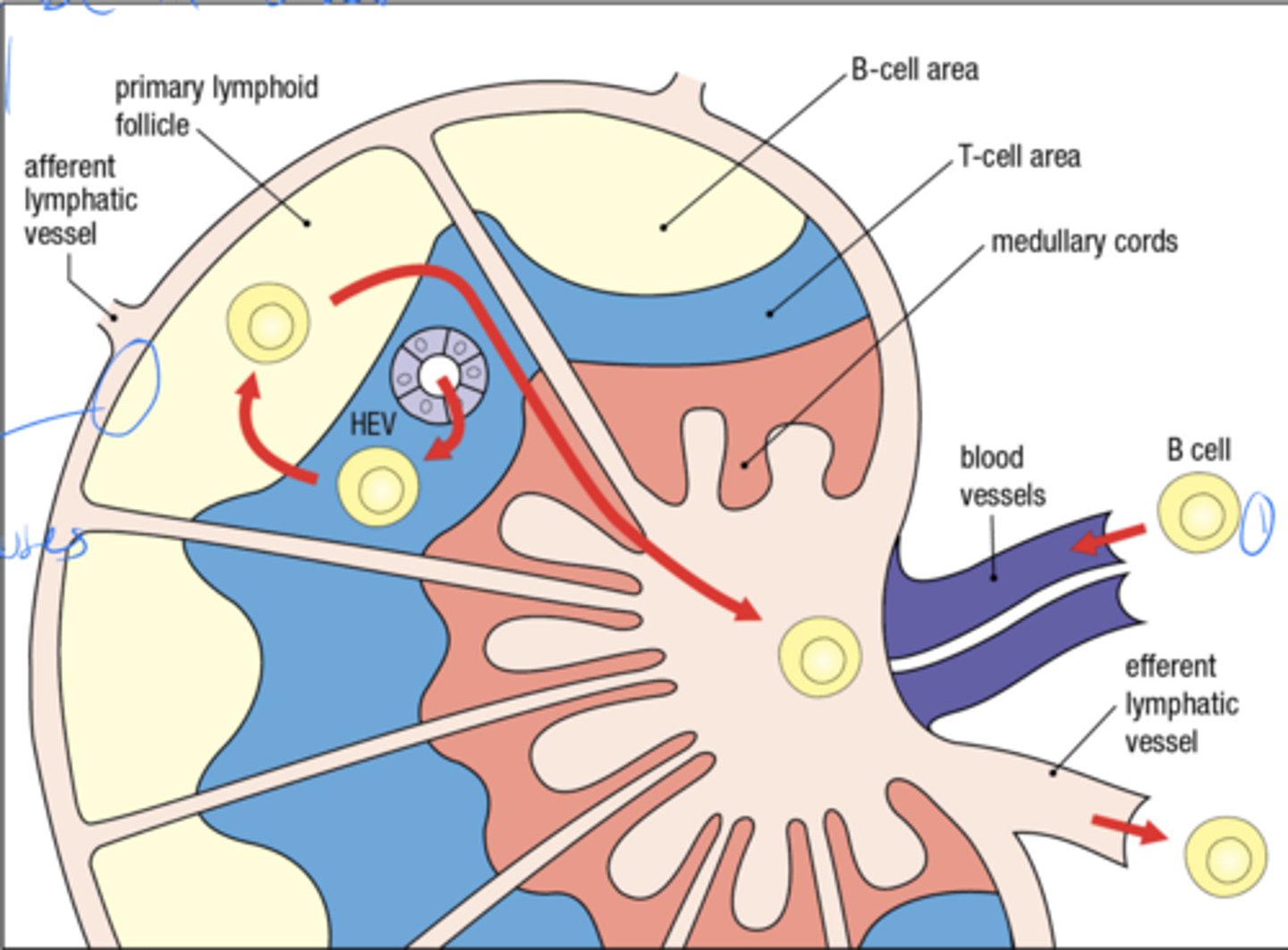
what is the anatomy of the lymph node
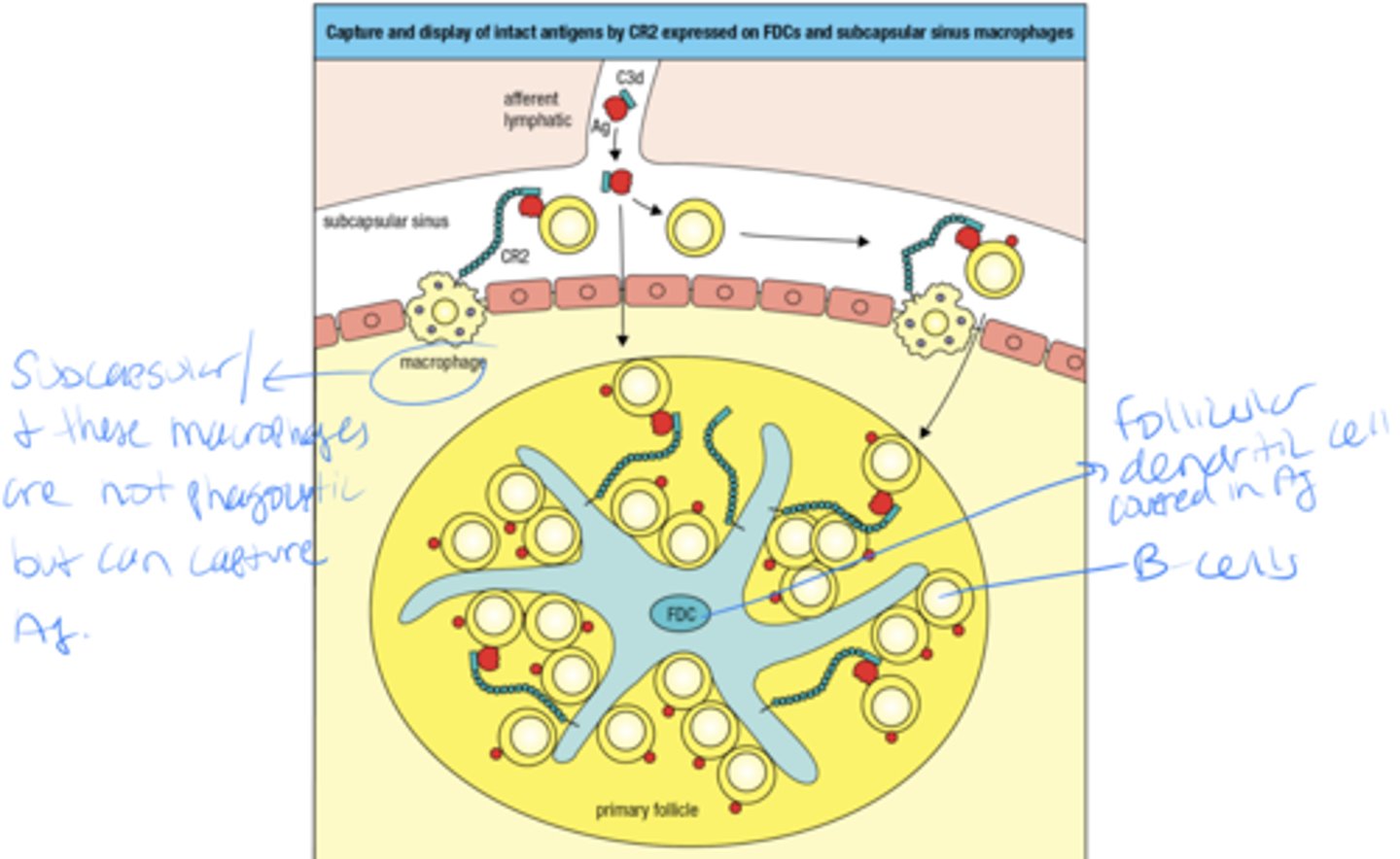
The B-cell needs to encounter what in order to be activated
B cell needs to encounter follicular dendritic cell in order for B cell to mature and develop
Follicular dendritic cells in the B-cell area store and display intact antigens to
B-cells
- capture and display of intact antigens by CR2 expressed on follicular dendritic cells and subcapsular sinus macrophages
Effective B cell-mediated immunity depends on help from
CD4 T cells
The population of B cells (B-1 cells) do not require
T cell help and do not undergo isotype switching and affinity maturation
B-1 cells need
B-1 Cells need to bind to multiple epitopes for stronger response
B-1 cells produce antibodies against
repetitive epitopes on pathogens – thymus-independent antigens (antigens that stimulate the B cells without help of T cells)
B-1 cells are
-fetal B-cells and they help protect the baby from infections
-B-1 cells have ability to talk to T cells and undergo isotype switching
Follicular dendritic cells in the B-cell area
store and display intact antigens to B cells
"homing of B cell to lymph node"
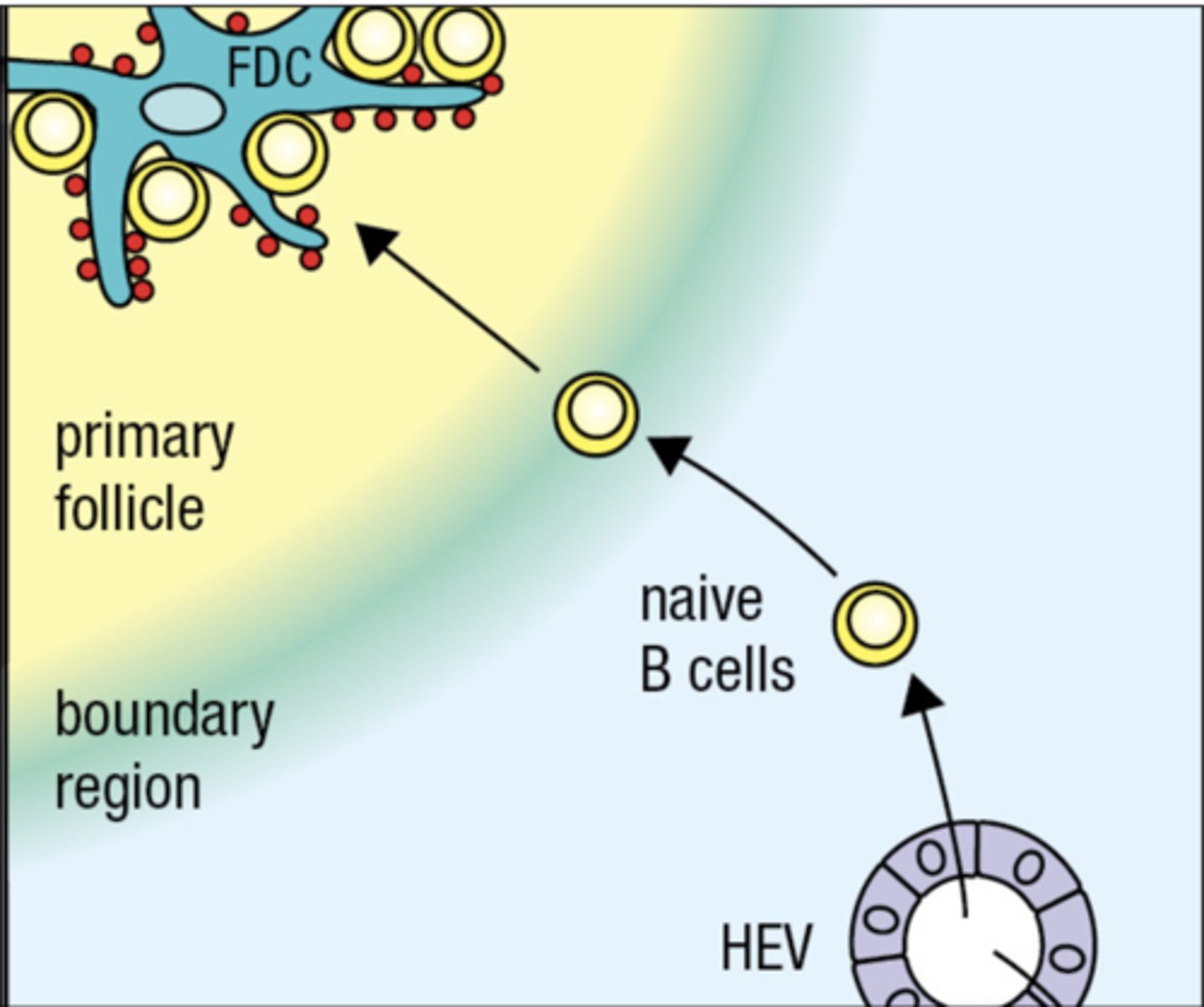
Antigen-activated B cells move close to the T-cell area to find a
helper TFH cell
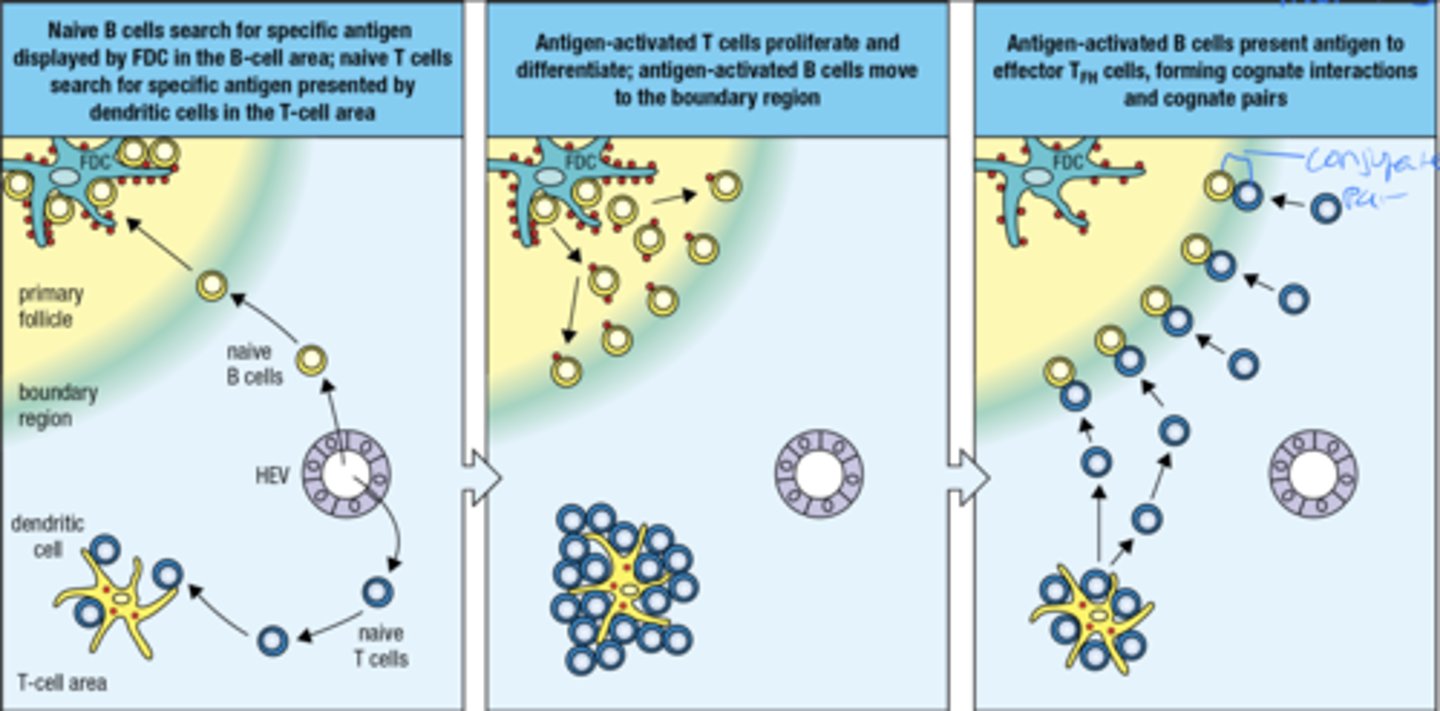
what is the 3 part process by which an antigen-activated B cell finds a TFH cell
1. naive B cells look for specific ag displayed by FDC in B cell area. naive t cells look for specific ag presented by dendritic cells in the t cell area
2. Ag-activated t cells proliferate and differentiate, ag-activated b cells move to the boundary region
3. Ag-activated B cells present ag to effector TFH cells, forming cognate interactions and pairs (CD40 helps form cognate pairs)
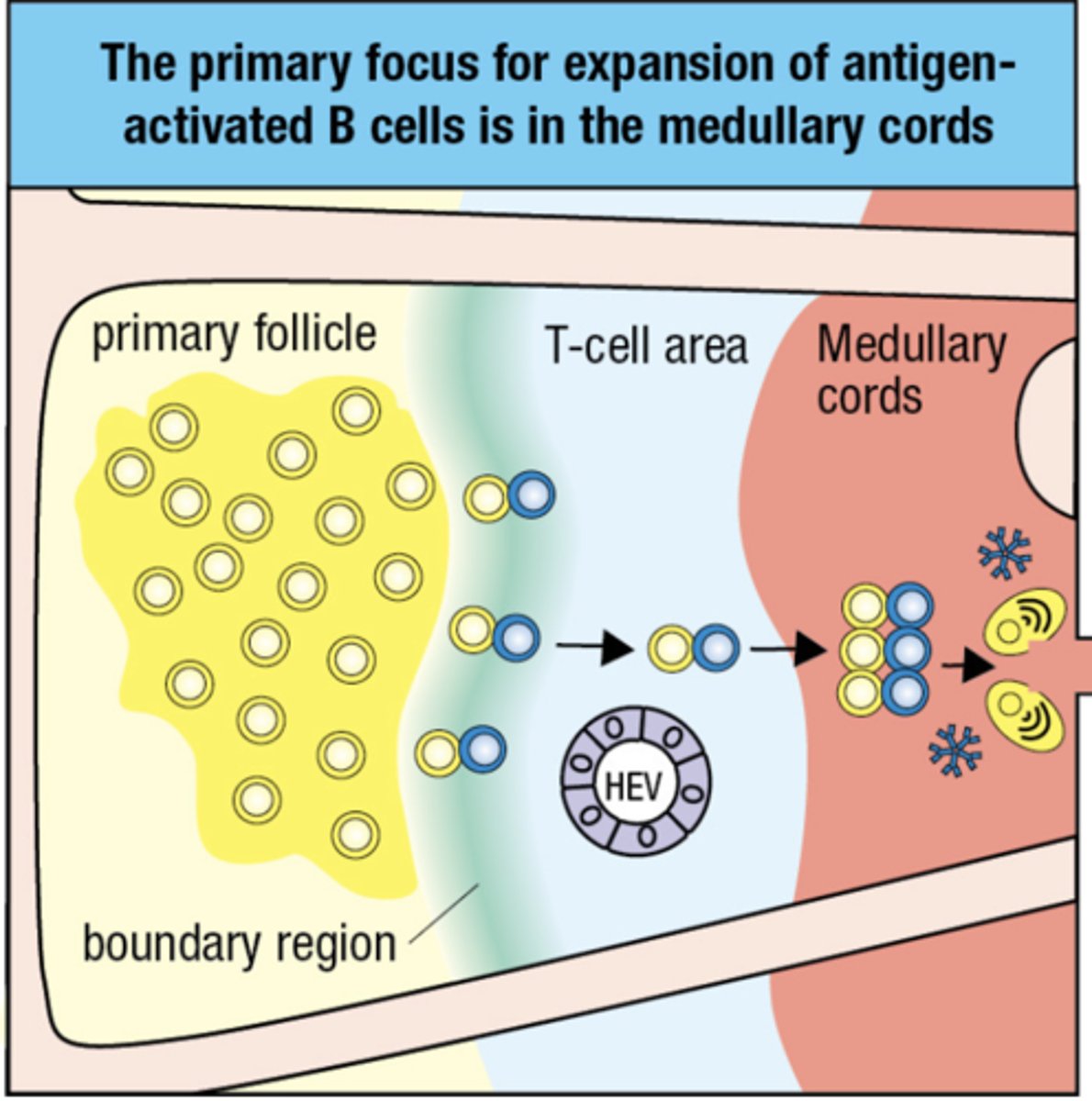
The primary focus of clonal expansion in the medullary cords produces
plasma cells secreting IgM
what is the second focus for expansion
the second focus is for expansion of ag- activated B cells in the primary follicle
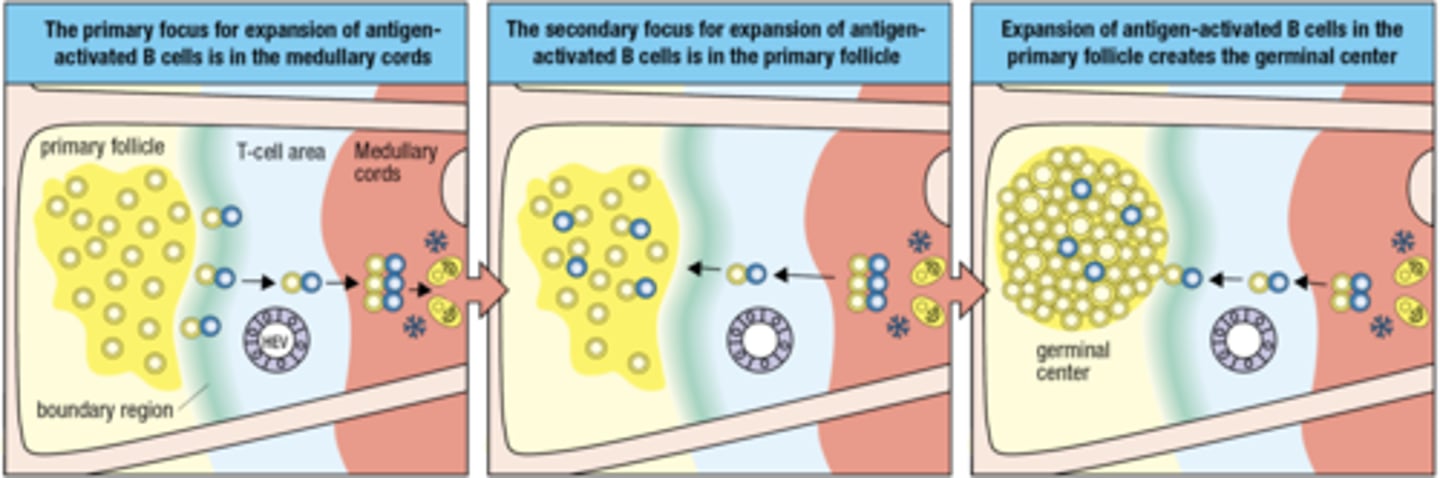
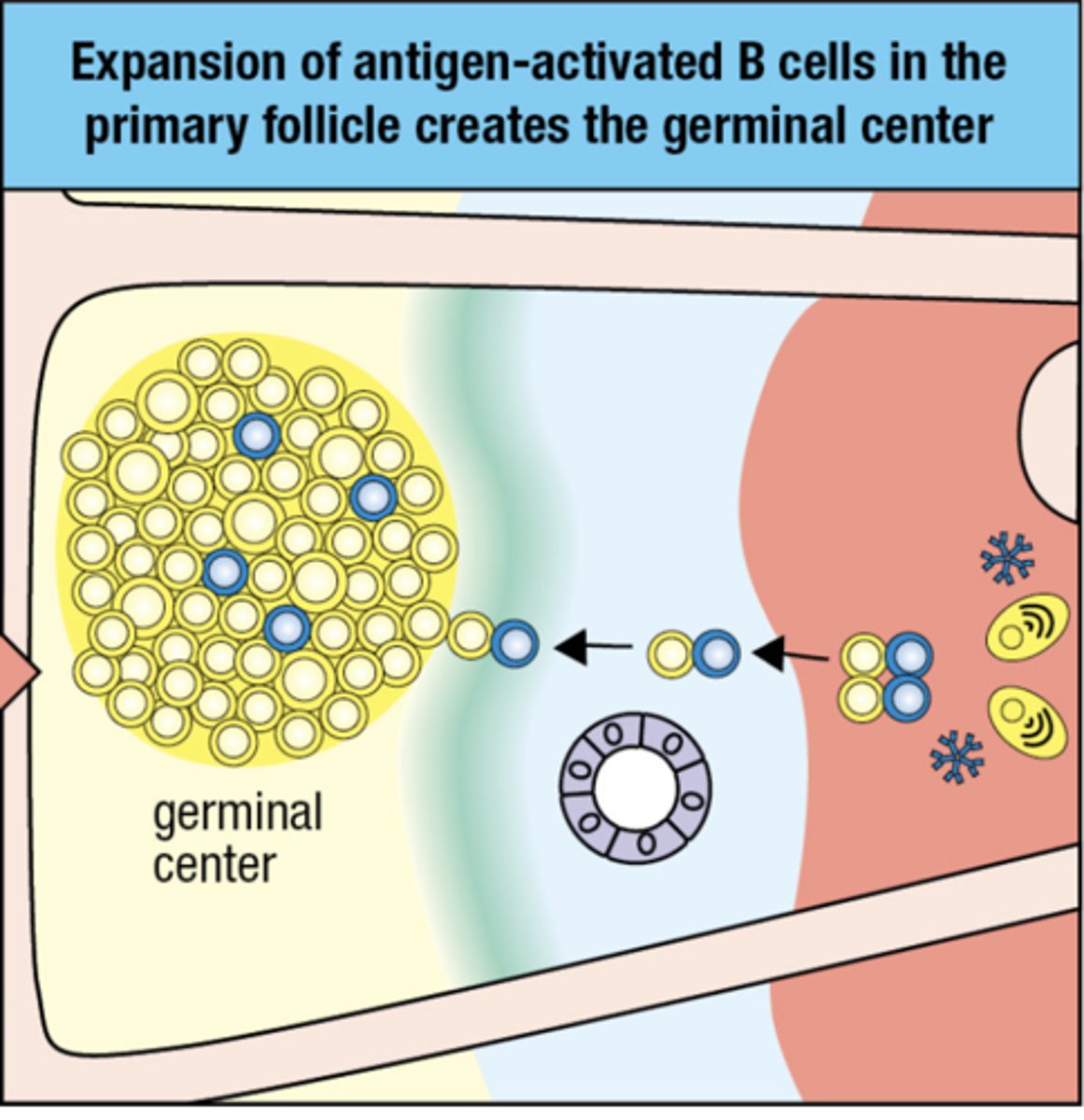
Expansion of antigen-activated B cells in the primary follicle creates the
germinal center
high amounts of IgM's at this stage means that there is an early infection
TFH helps B cells to proliferate by secreting what
IL-5 and IL-6
what causes swelling of lymph nodes
Germinal centers
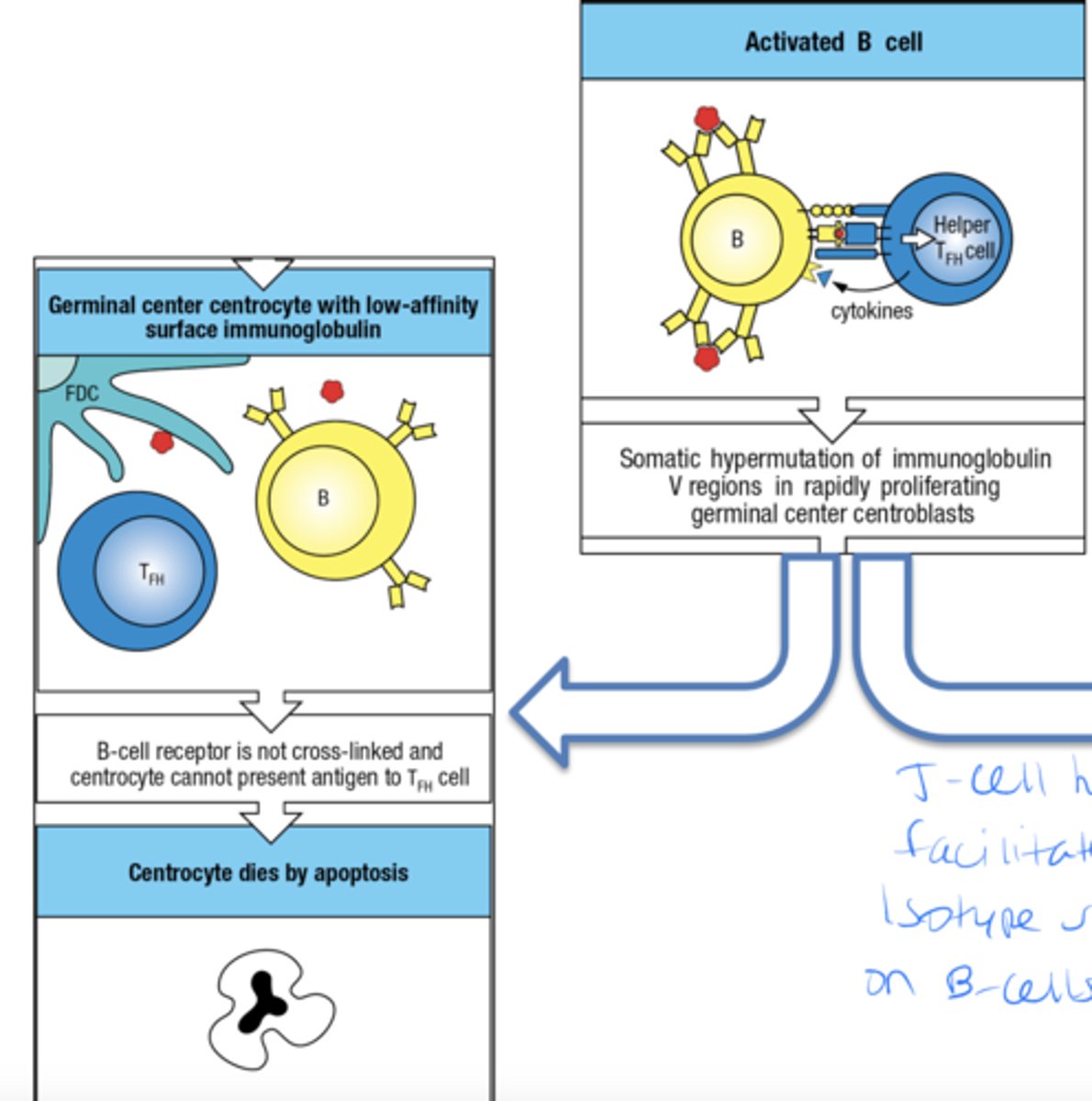
once the B cell is activated what is one pathway they can take
- B cell receptor is not cross linked and centrocyte (B cell) cannot present ag to TFH
- centrocyte dies by apoptosis
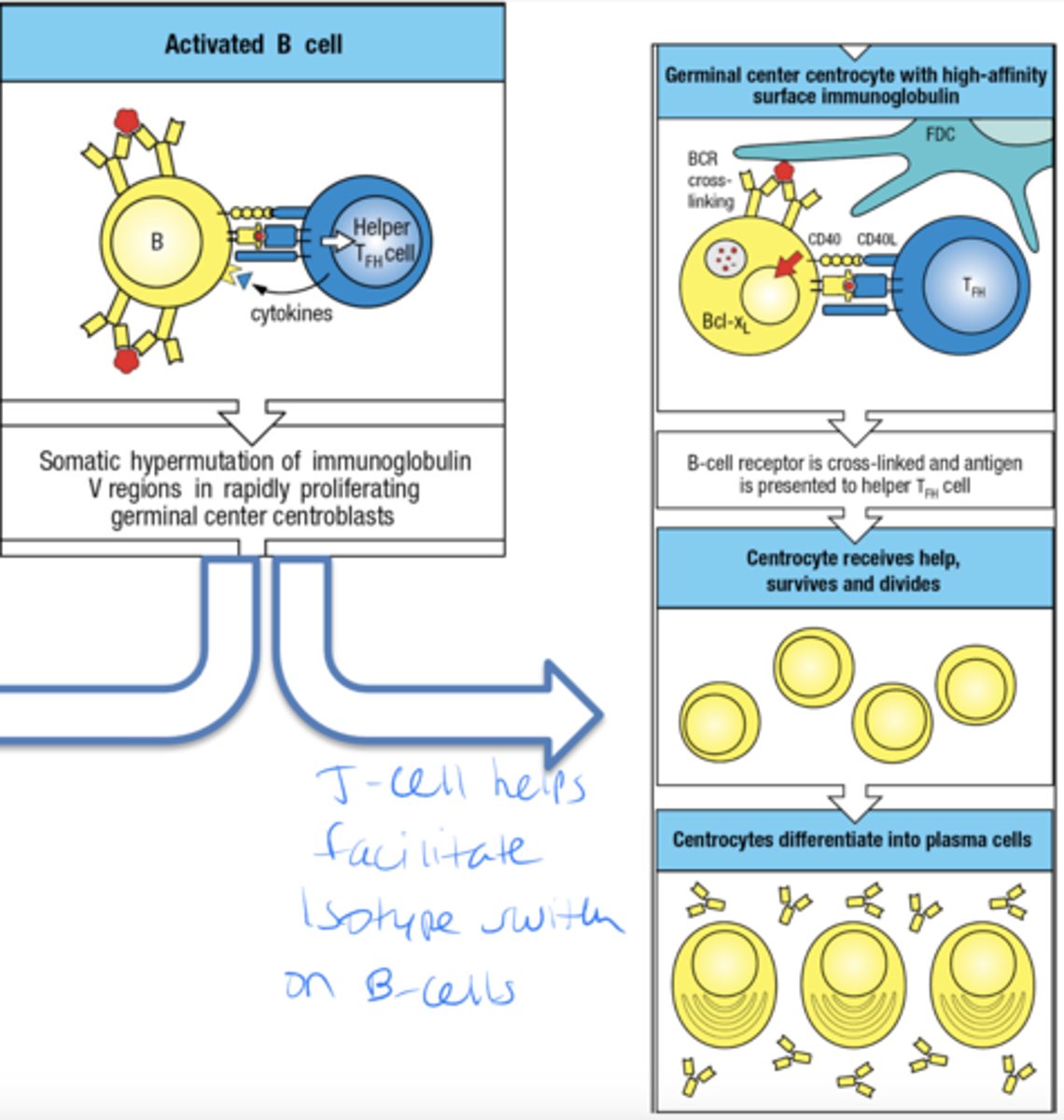
once the B cell is activated what is the other pathway they can take
- B cell receptor is cross-linked and ag is presented to helper TFH
- centrocyte receives help, survives and divides
- centrocytes differentiate into plasma cells
Isotype switching takes place mainly within the
germinal center
The isotype is determined by its interactions with
TFH cell
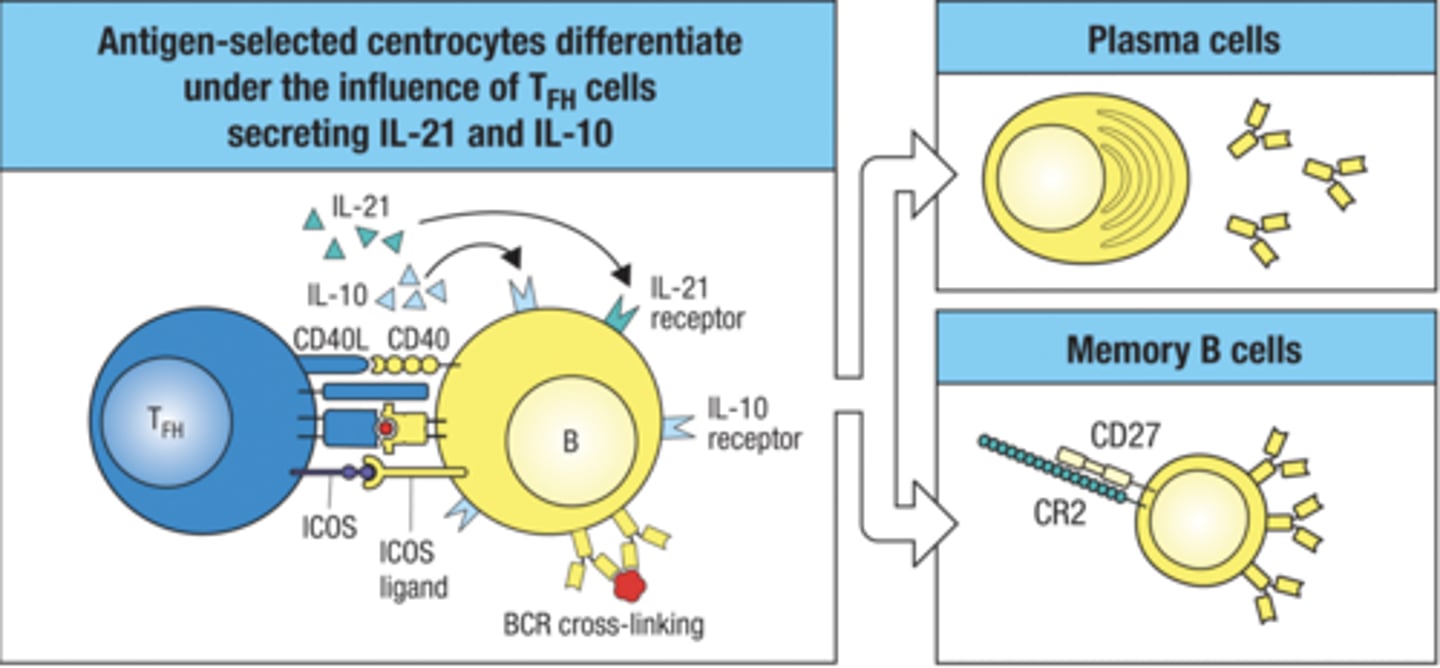
TFH cells determine the differentiation of antigen- activated B cells into plasma cells or memory cells. what is the process
- antigen selected centrocytes differentiate under the influence of TFH cells secreting IL-21 and IL-10
- then it develops into memory or plasma cells
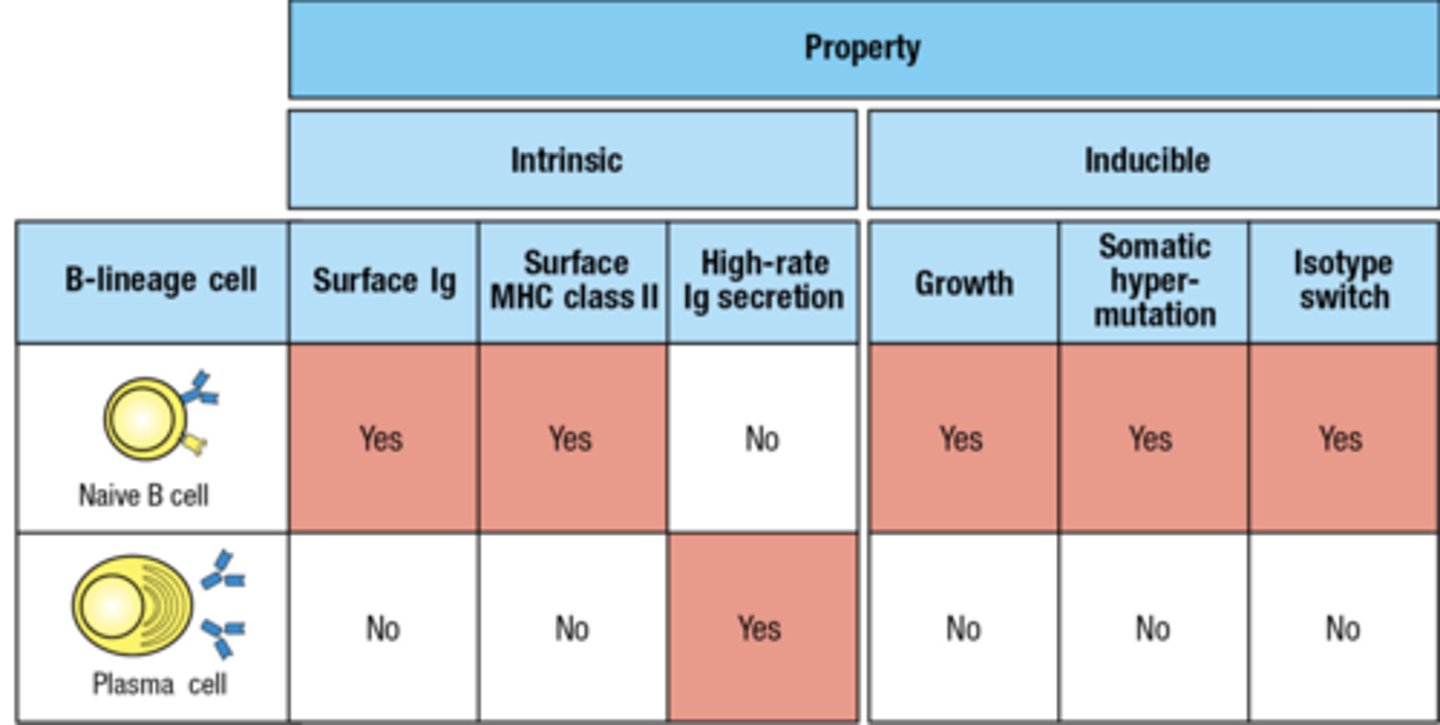
look at this chart
love this chart
Antibodies with different C regions have different
effector functions
also look at this chart and focus on the categories that have the arrows
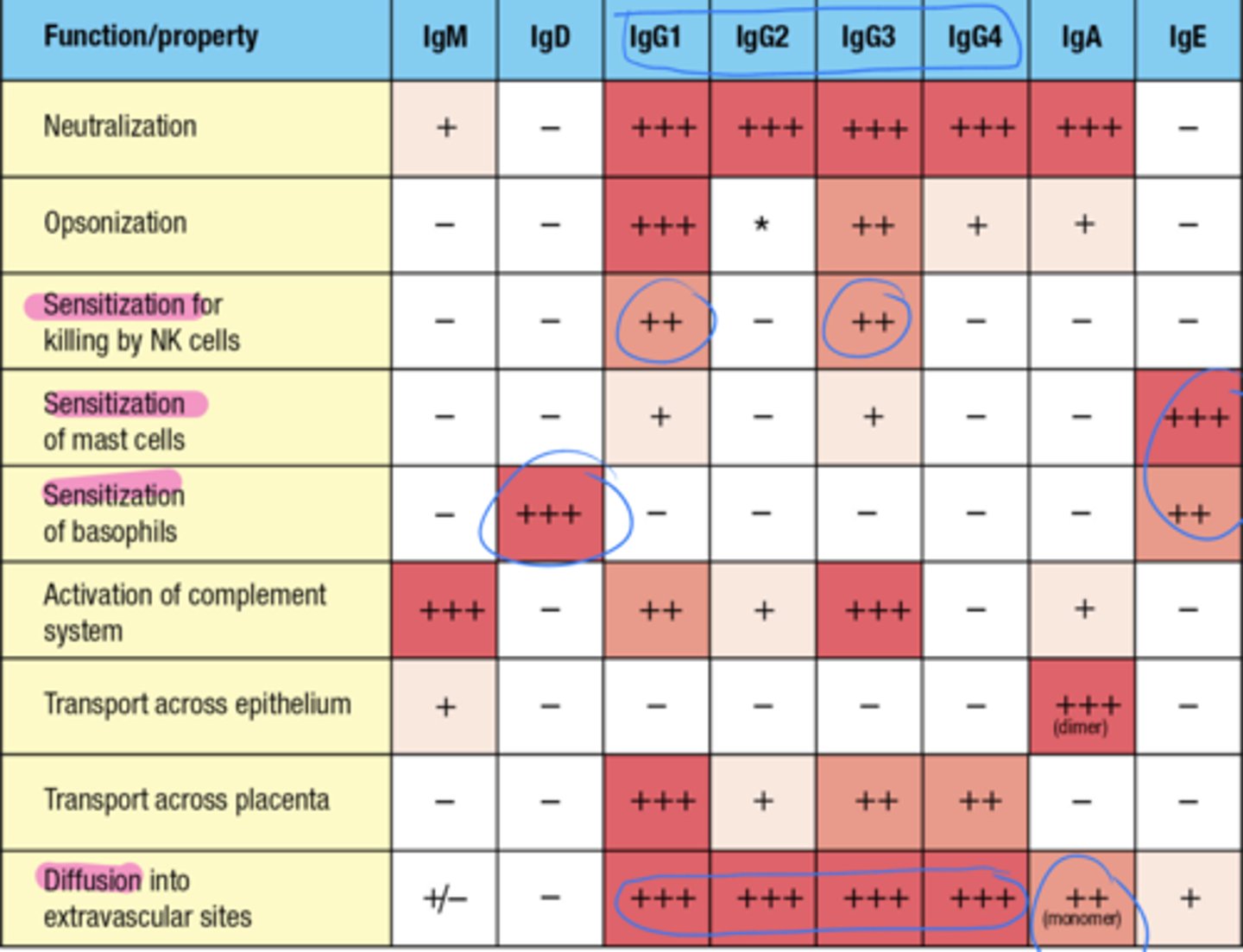
which Ig's have the function of activating the complement system
IgM and IgG3
which Ig's have the function of transporting across the epithelium
IgA
which Ig's have the function of transporting across the placenta
IgG1
what 3 Ig's provide a defense to all the tissues reached by the blood and prevent blood-borne infections
Circulating IgM, IgG, and IgA
The receptor FcRn transports what
IgG from the bloodstream into the extracellular spaces of tissues
The delivery of IgG to tissues is facilitated by
active transport from the blood into the extracellular spaces within tissues.
while associated with FcRn transport receptor IgG's are what
IgGs are pinocytosed by endothelial cells
FcRn protects IgGs from
degradation in lysosomes
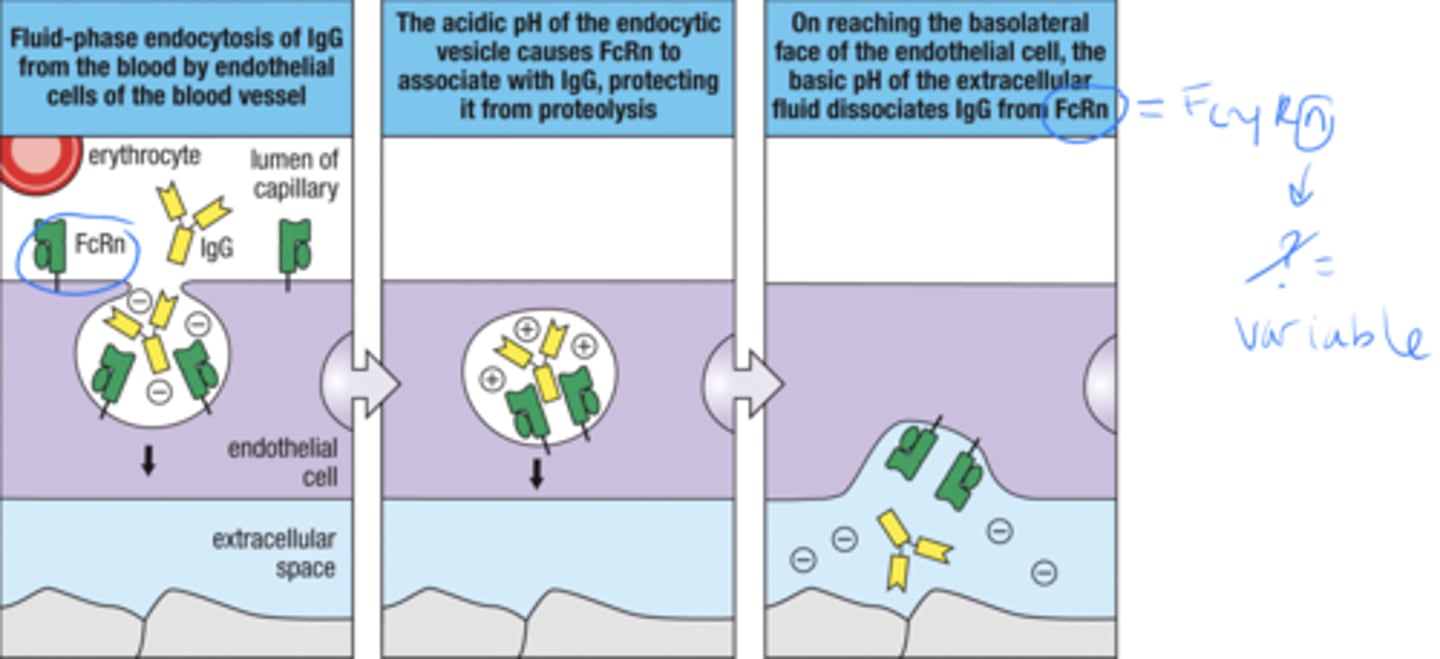
what is the 3 part fluid phase endocytosis of IgG
1. endocytosis of IgG from the blood by endothelial cells of the blood vessel
2. the acidic pH of the endocytic vesicle causes FcRn to associate with IgG, protecting it from proteolysis
3. on reaching the face of the endothelial cell, the basic pH of the extracellular fluid disassociates IgG from FcRn
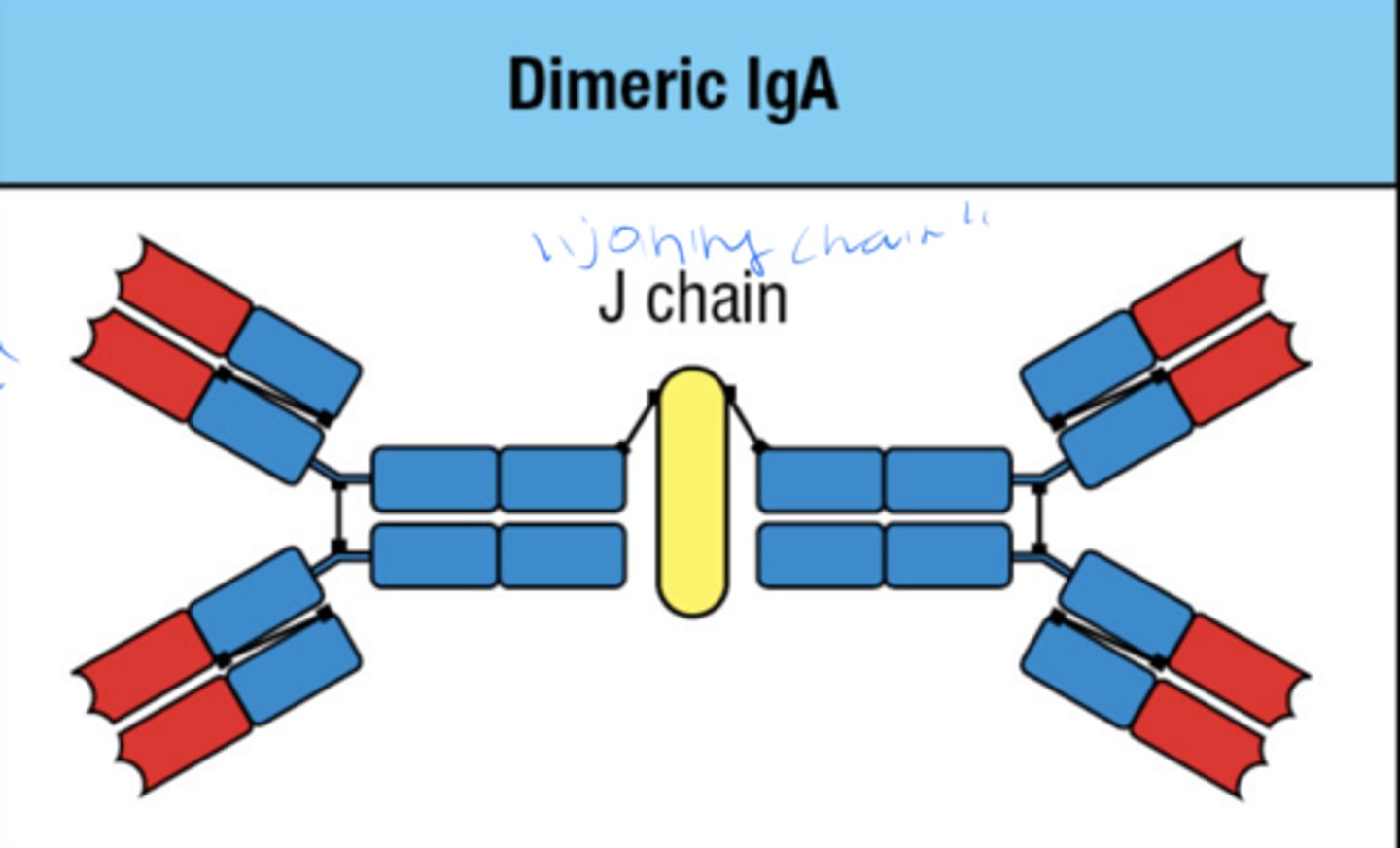
Dimeric IgA protects the surfaces of the mucosal epithelia that -
interact with the external environment
GI, eyes, nose, throat, respiratory, urinary, genital tracts, mammary glands
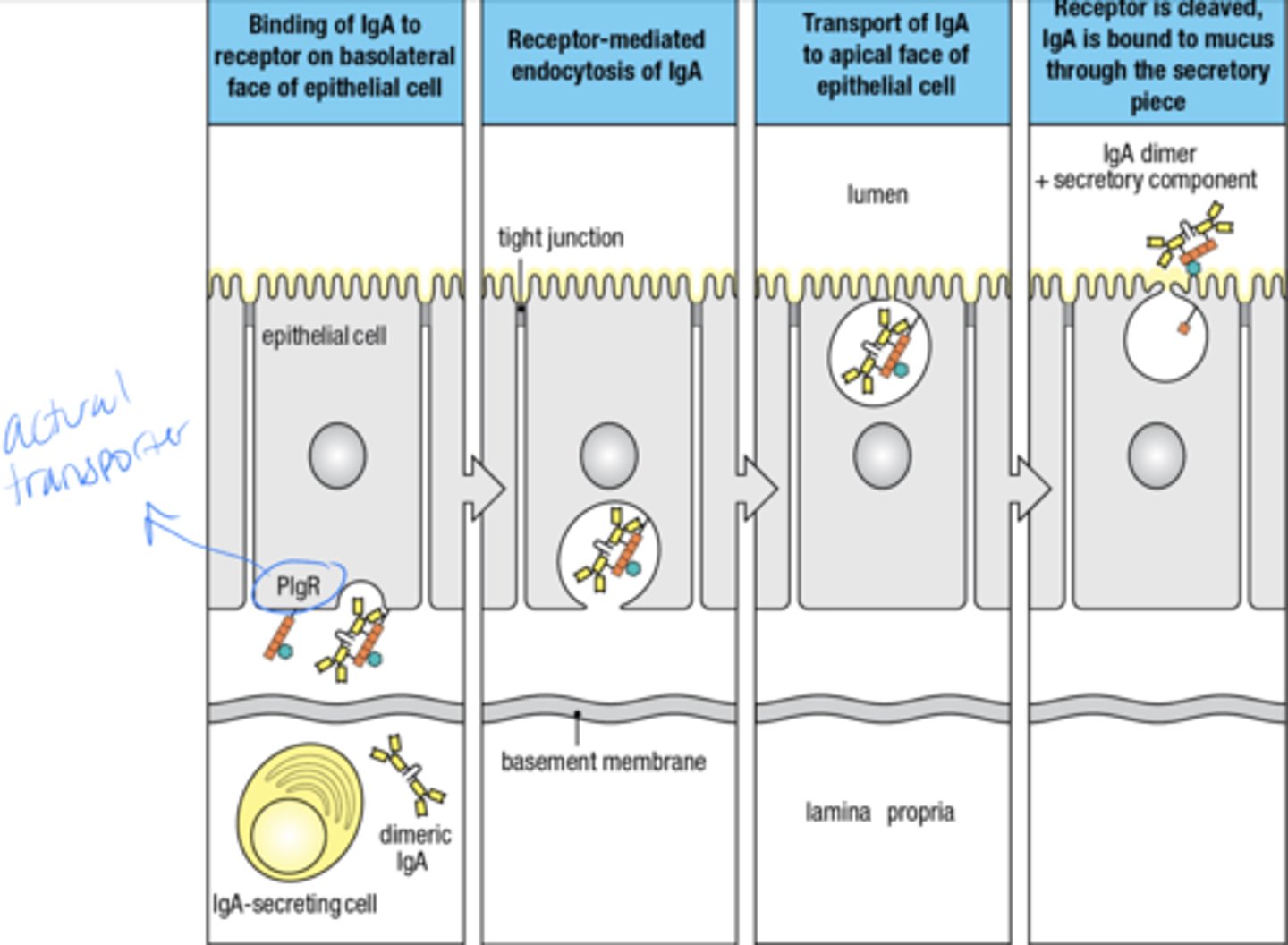
what is the 4 step process by which IgA protects the mucosal surfaces of the body
1. binding of IgA to receptor on basolateral face of epithelial cell
2. Receptor mediated endocytosis of IgA
3. transport of IgA to epithelial cell
4. receptor is cleaved, IgA is bound to mucus through the secretory piece
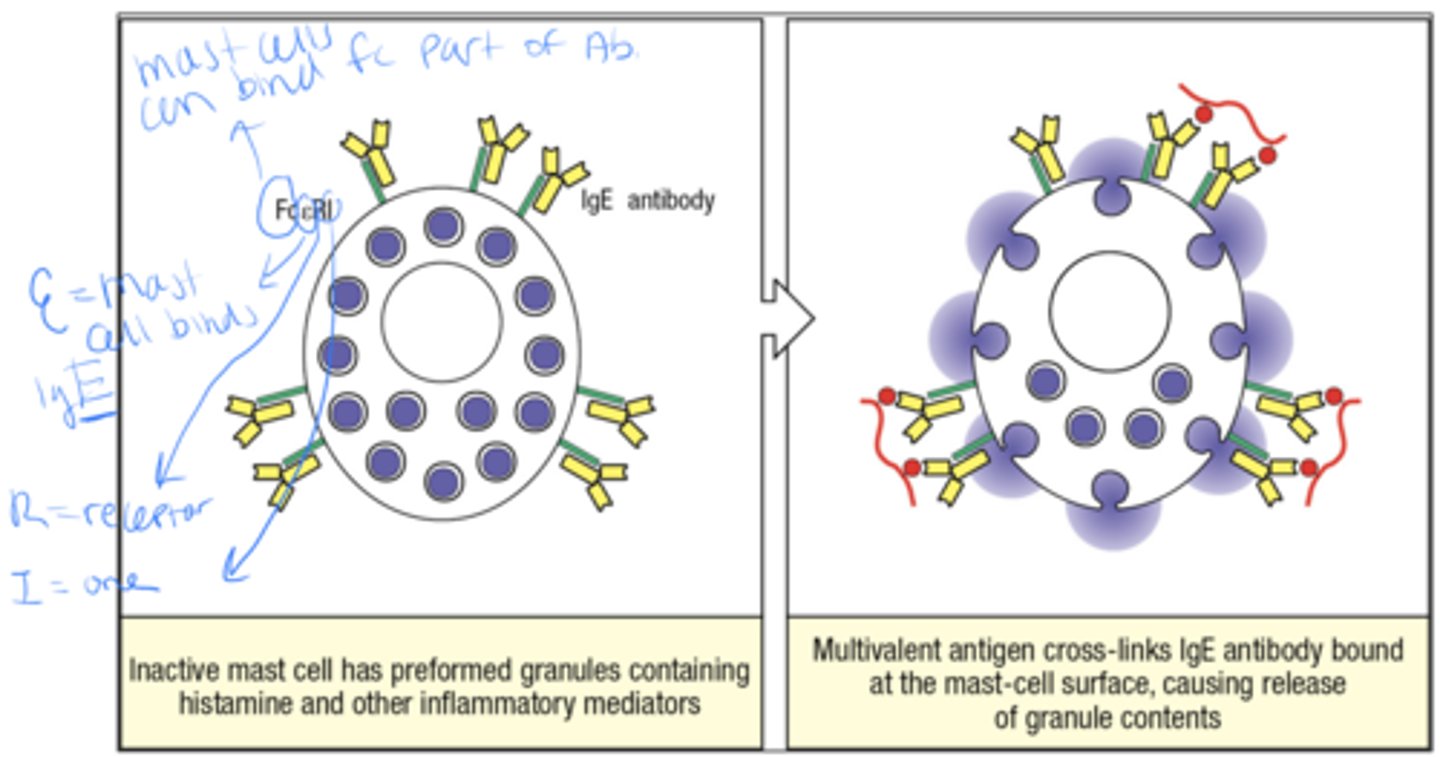
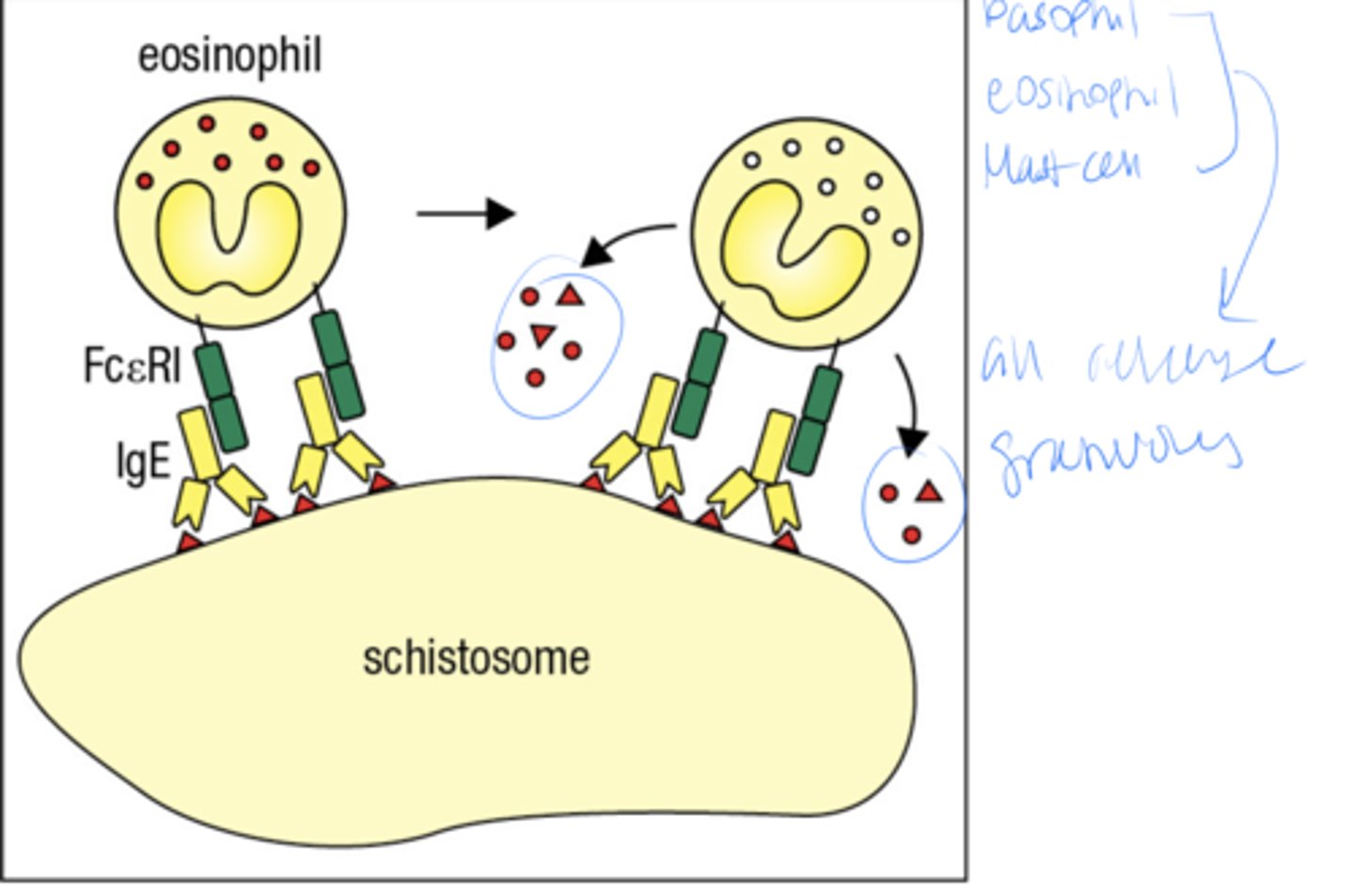
what is the 2 part mechanism by which IgE ejects parasites from the body
1. Inactive mast cell as preformed granules containing histamine and other inflammatory mediators
2. Multivalent Ag Cross-links IgE Ab bound at the mast cell surface, causing release of granule contents
A schistosome is what
a type of parasitic worm basically
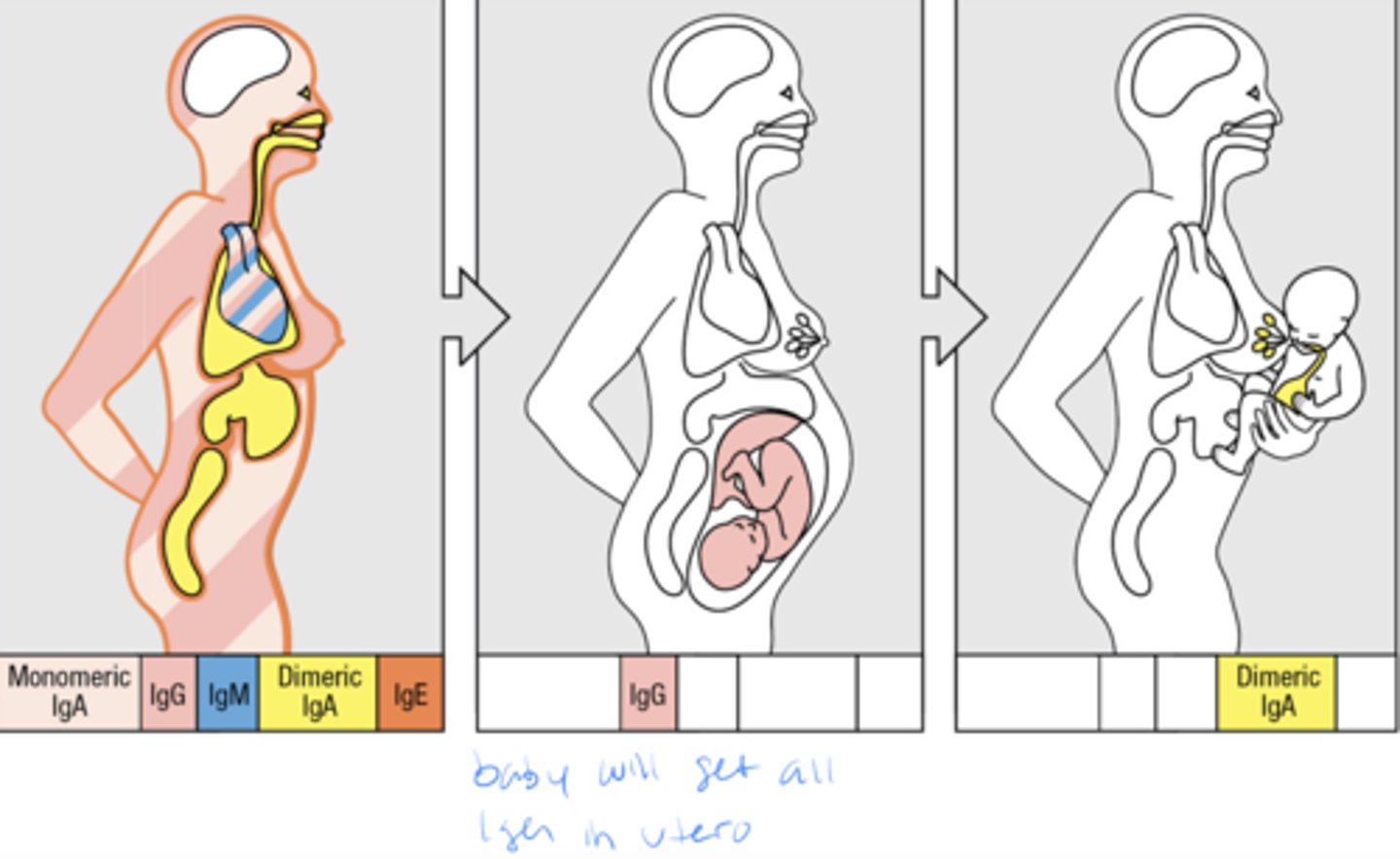
Mothers provide protective antibodies to their young, both before and after birth
passive immunity= baby got abs from mom so its own immune system was not activated therefore baby will lose all those ab shortly after birth, during breastfeeding baby will get some antibodies as well
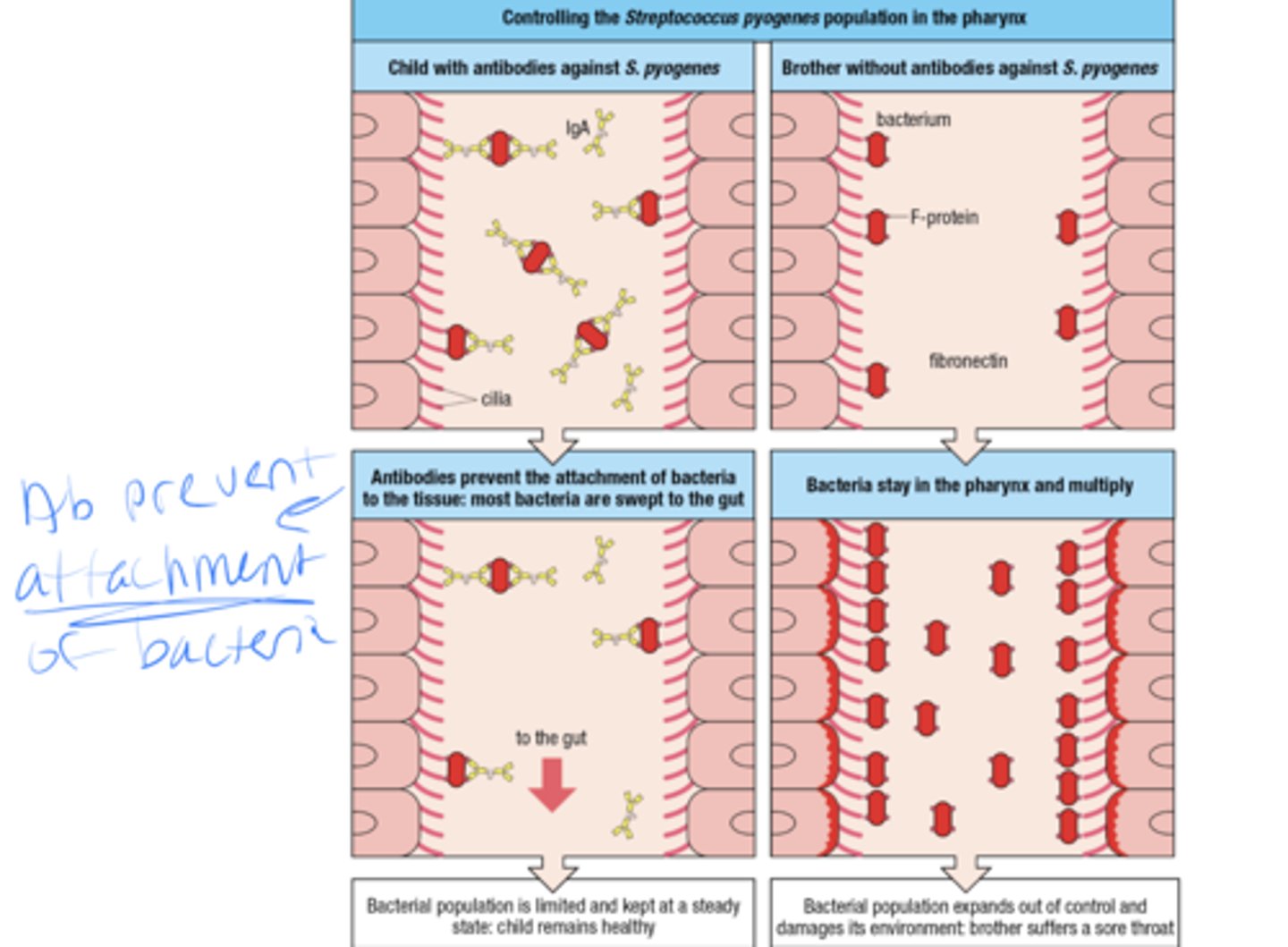
High- affinity neutralizing ab prevent bacteria from infecting cells, how does this work
-if the child has abs against strep, the ab will prevent the attachment of bacteria to the tissues and most bacteria get swept to the gut
- if the child does not have abs against strep then the bacteria stay in the pharynx and multiply
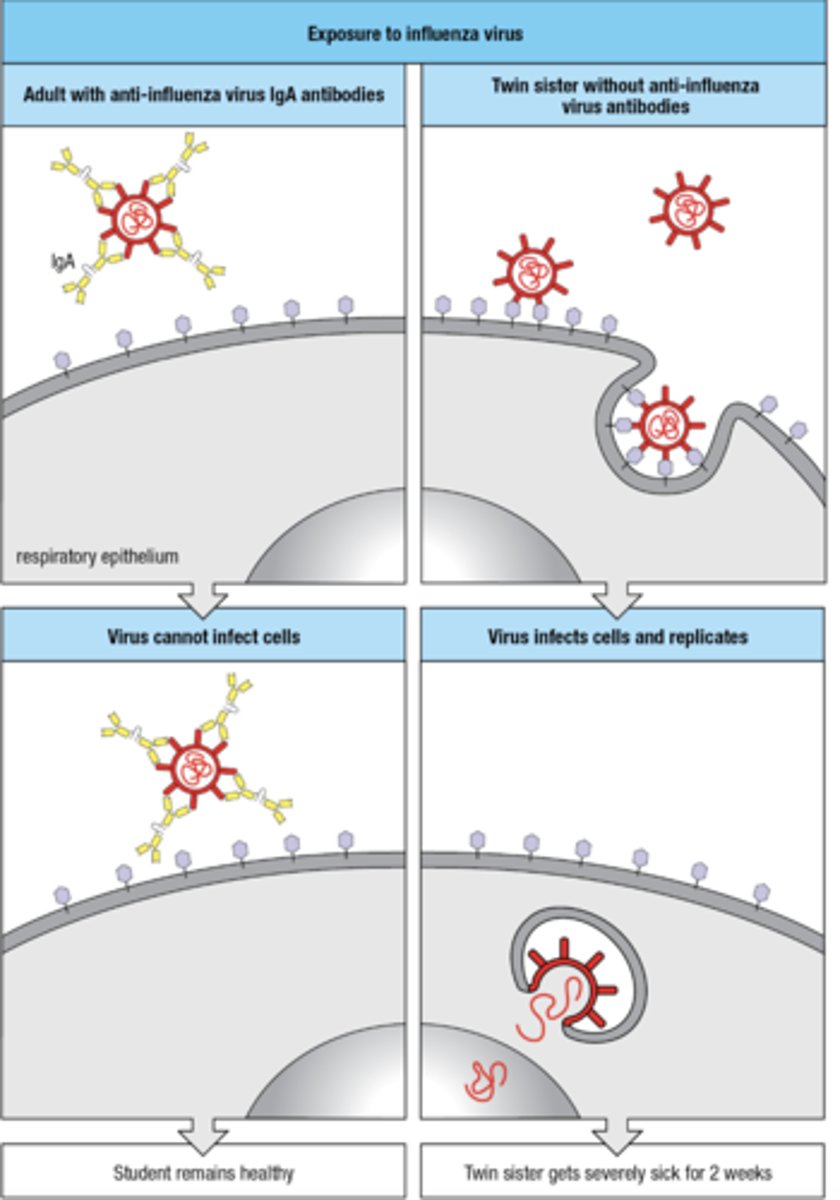
High- affinity neutralizing ab prevent viruses from infecting cells, how does this work
- person with anti-flu IgA ab, the virus cannot infect the cells
- person without antibodies, virus infects cells and replicates
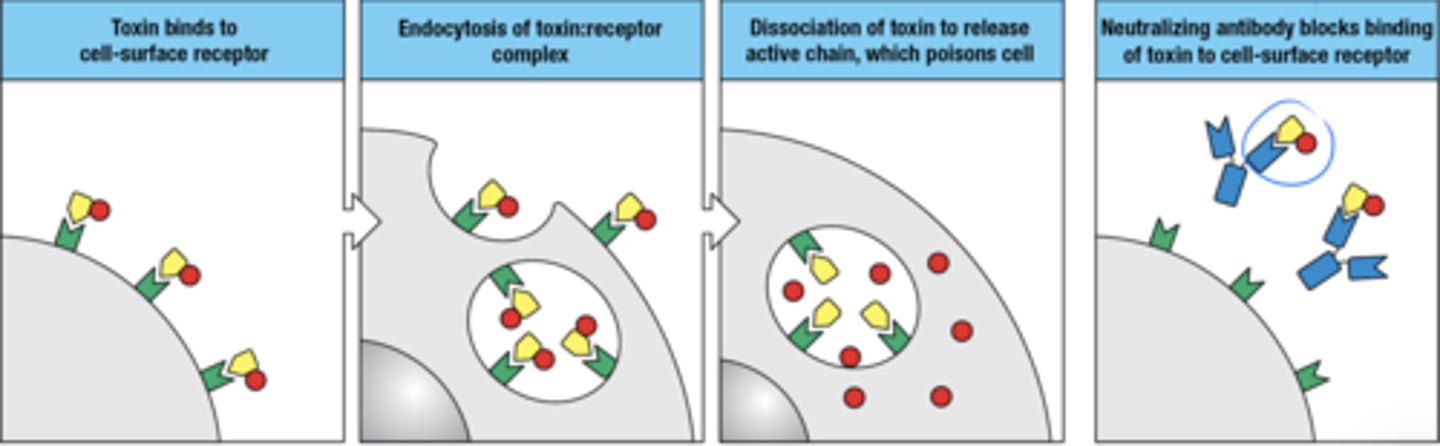
How are IgG and IgA antibodies used to neutralize microbial toxins and animal venoms
1. toxin binds to cell surface receptor
2. endocytosis of receptor:toxin complex
3. toxin disassociates to release active chain which poisons cell
4. neutralizing ab blocks binding of toxin to cell surface receptor
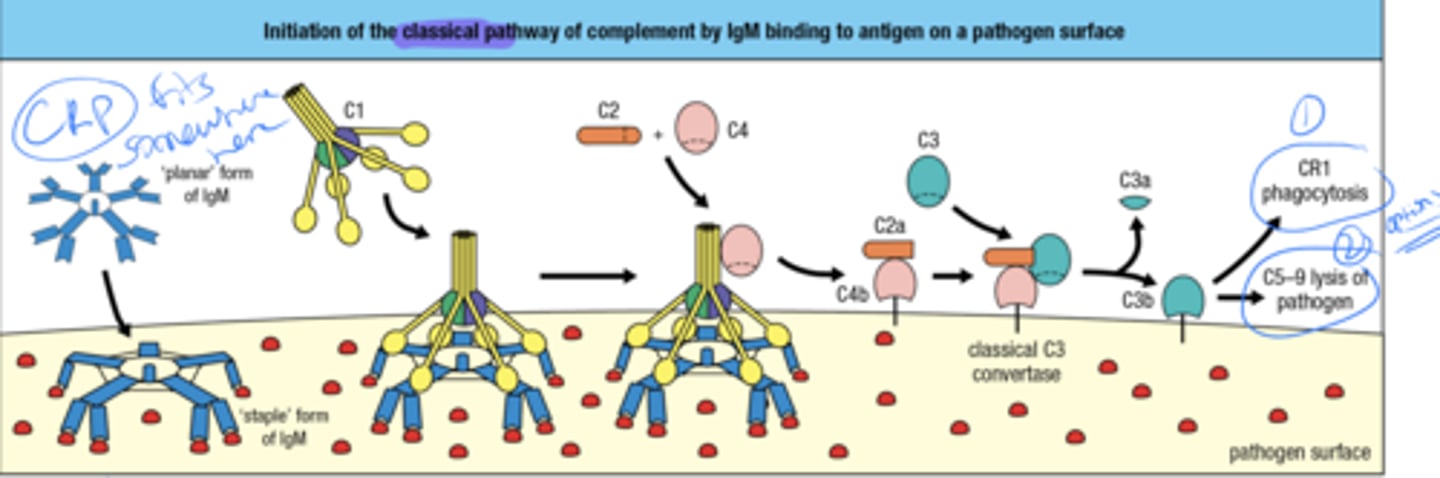
what initiates the complement by the classical pathway
Binding of IgM to antigen on a pathogen's surface
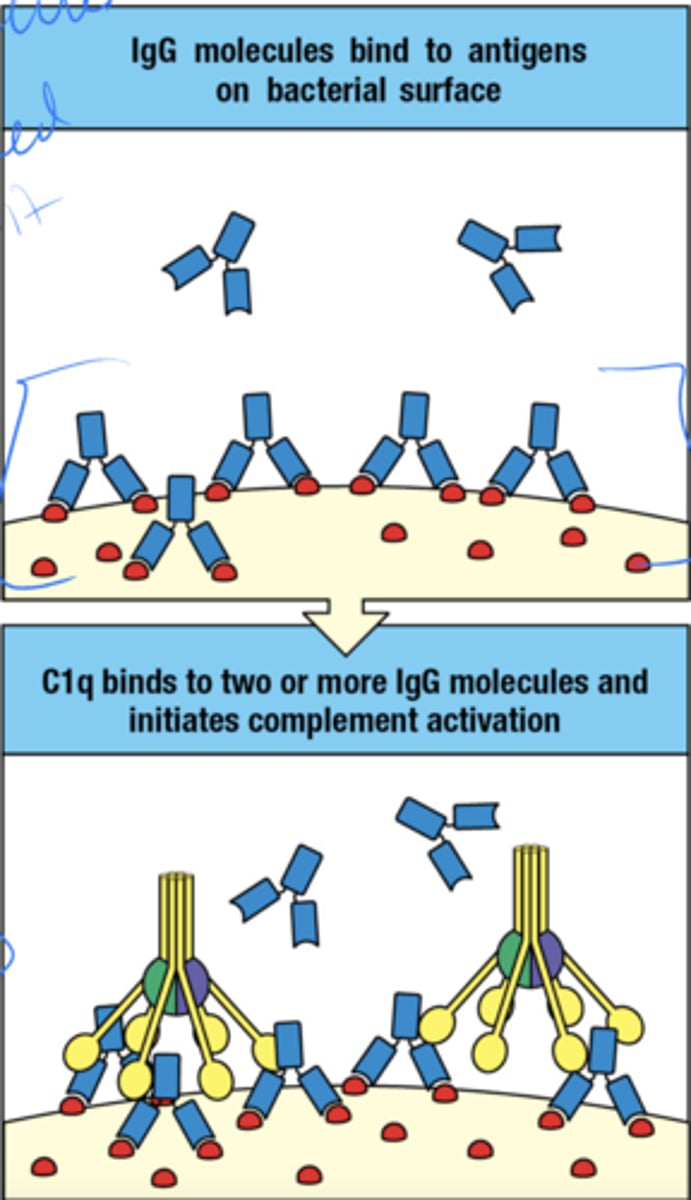
these Ig's are made after affinity maturation and isotype switching so what does that mean for the binding strength
ig binds very strongly to ag
how is complement activated by IgG when the ag's are on bacterial surface
- multiple molecules are needed to bind to the ag on the surface
- C1q binds to 2 or more IgG molecules and complement is activated
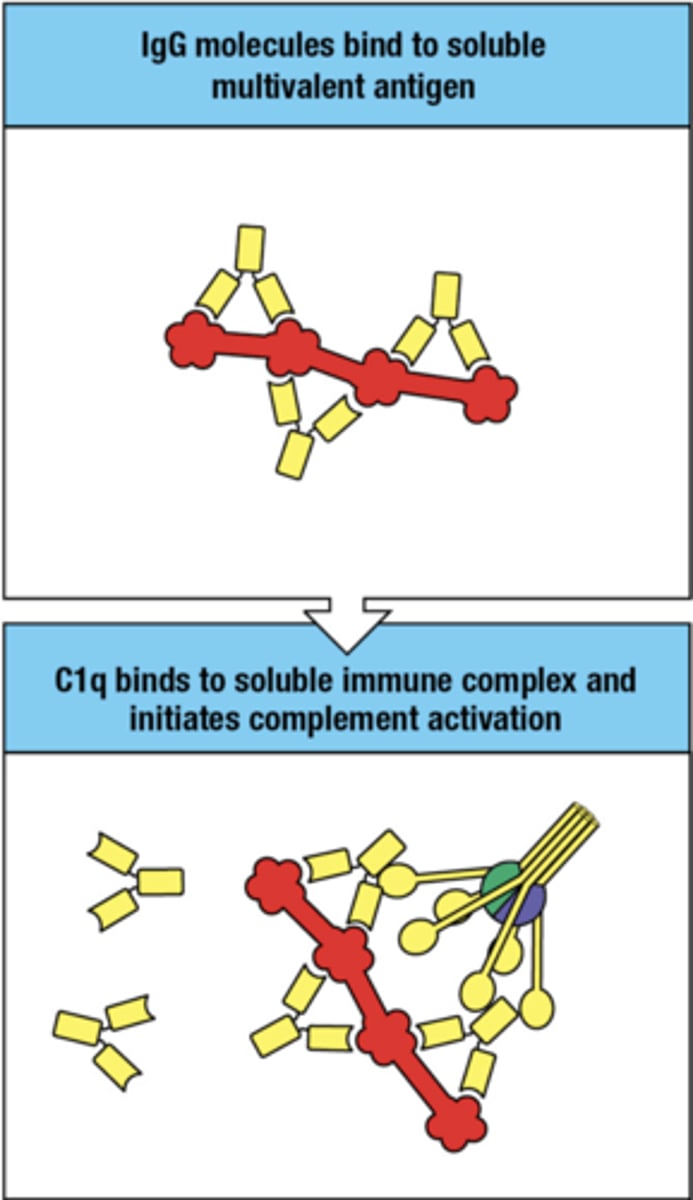
how is complement activated by IgG when the ag's are soluble multivalent ag's
- IgG bind to soluble multivalent ag
- C1q binds to IgG molecules and complement is activated
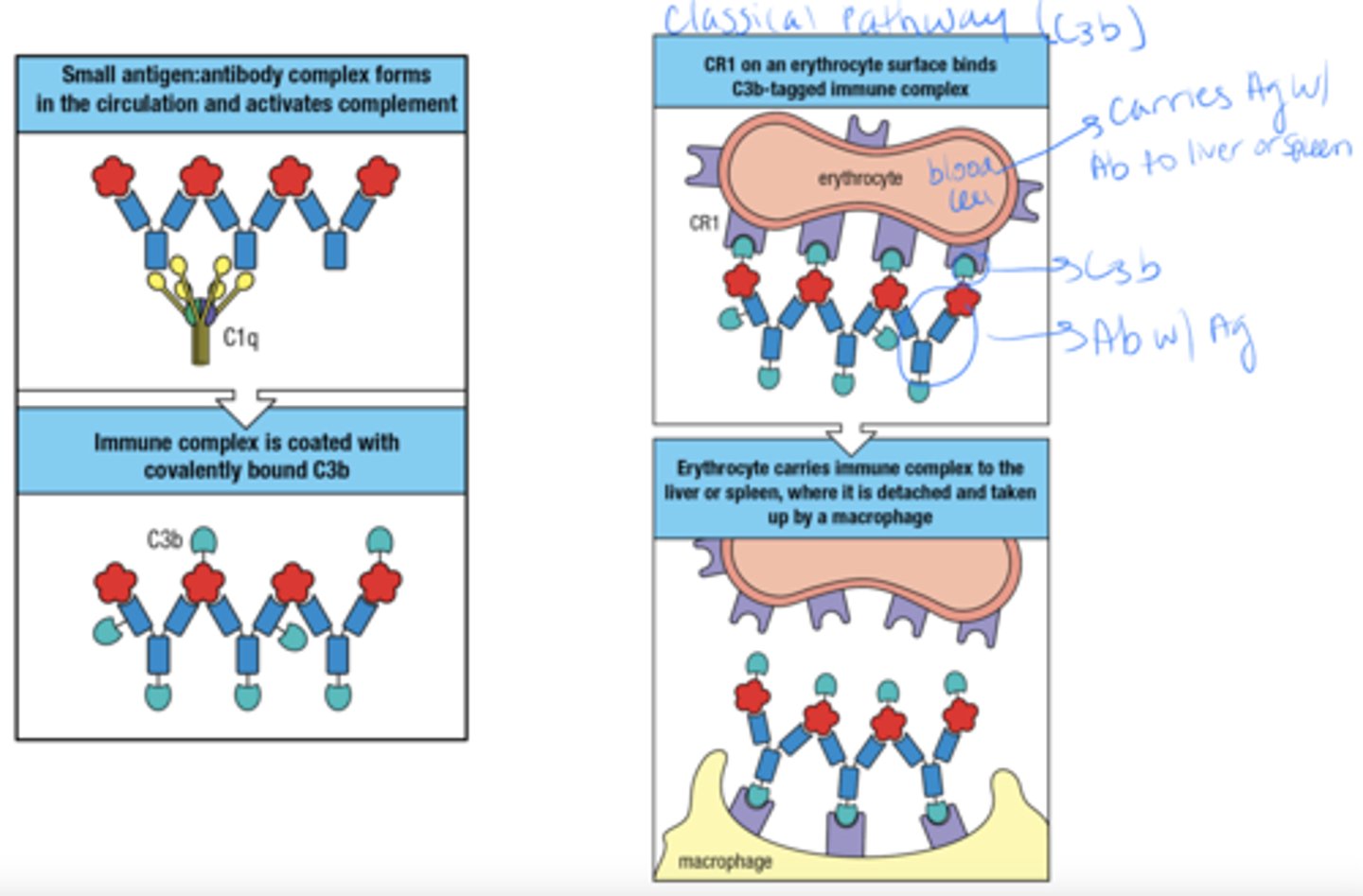
what type of cell can facilitate the removal of immune complexes from the circulation
Erythrocytes
how can Erythrocytes facilitate the removal of immune complexes from the circulation
- ag:ab complex forms and triggers complement
- complex is covered with C3b
- CR1 from Erythrocyte binds to the C3b tagged complex
- Erythrocyte carries complex to liver or spleen where a macrophage then deals with it
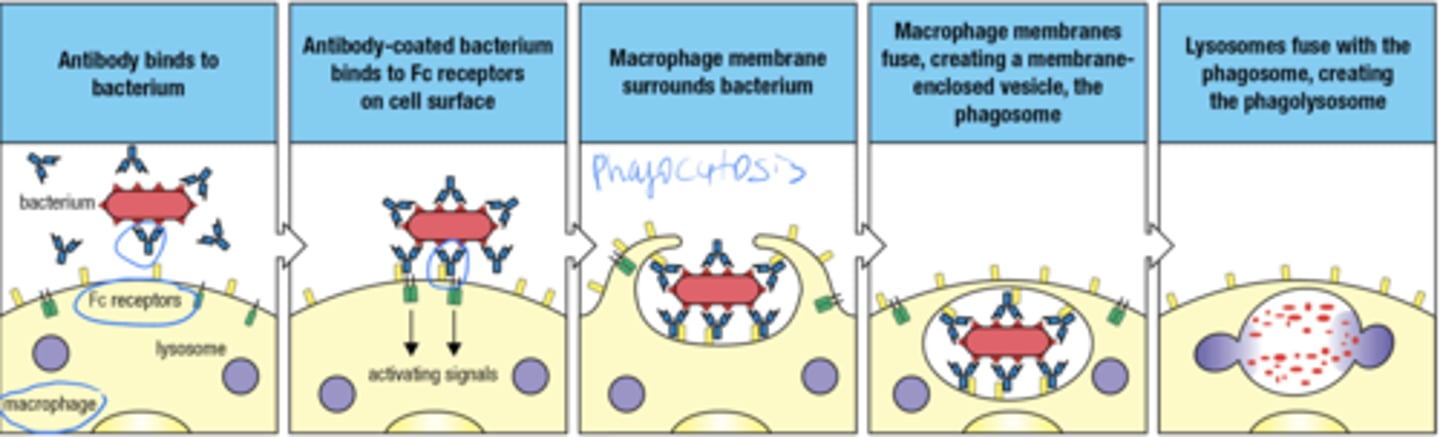
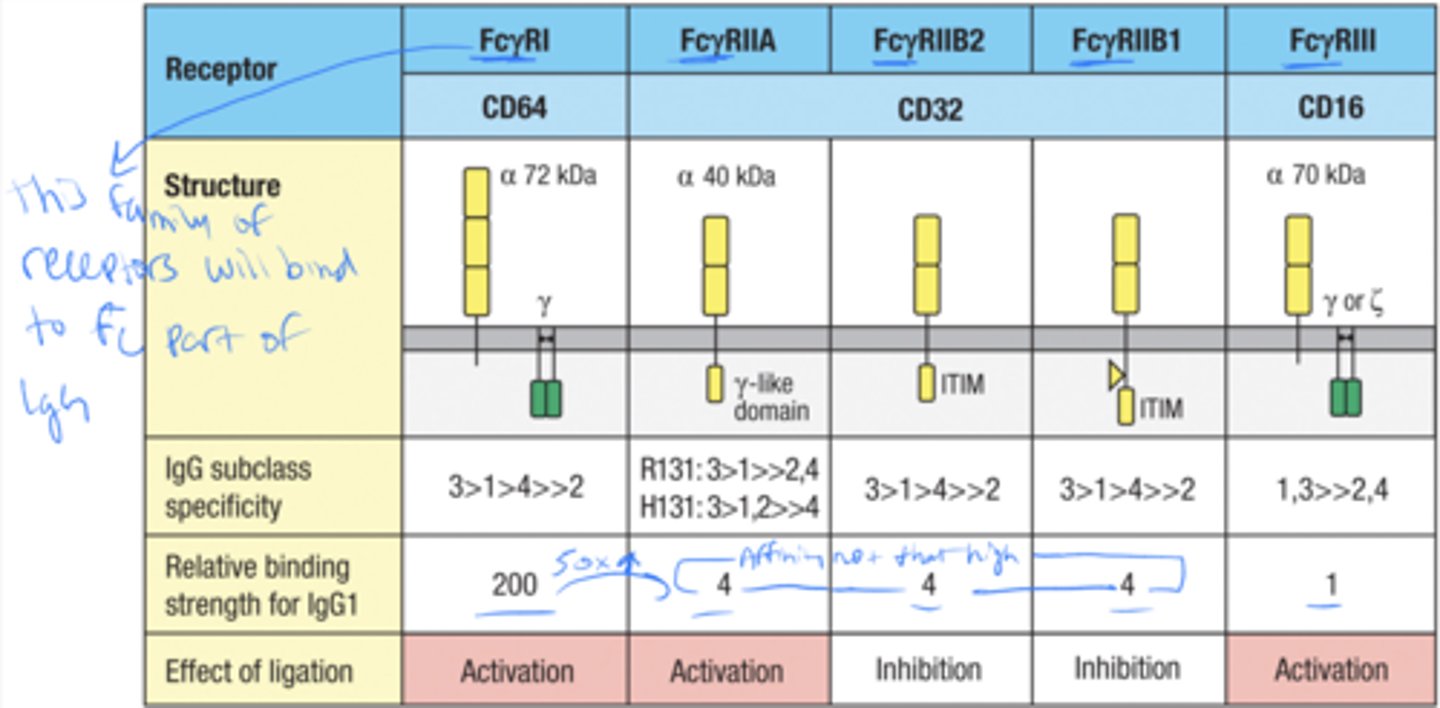
Fcγ receptors enable effector cells to bind and be _____
activated by IgG bound to pathogens
what Fc receptor is specific for IgG
Fc(gamma)RI
Fc(gamma)RI is constitutively expressed by
monocytes, macrophages, and DC
FcgRI facilitates the
uptake and degradation of pathogens by phagocytes and professional APCs
what is the 5 step process by which FcgRI facilitates the uptake and degradation of pathogens by phagocytes and professional APCs
- ab binds to bacterium
- Ab coated bacterium binds to Fc receptors on surface
- macrophage membrane surrounds bacterium (phagocytosis)
- macrophage membranes fuse, forming the phagosome
- lysosomes fuse with phagosome forming phagolysosome
Activating receptors promote the
uptake and destruction pathogens
what are the 2 inhibitory Fc receptors
Fc(gamma)RIIB1
Fc(gamma)RIIB2
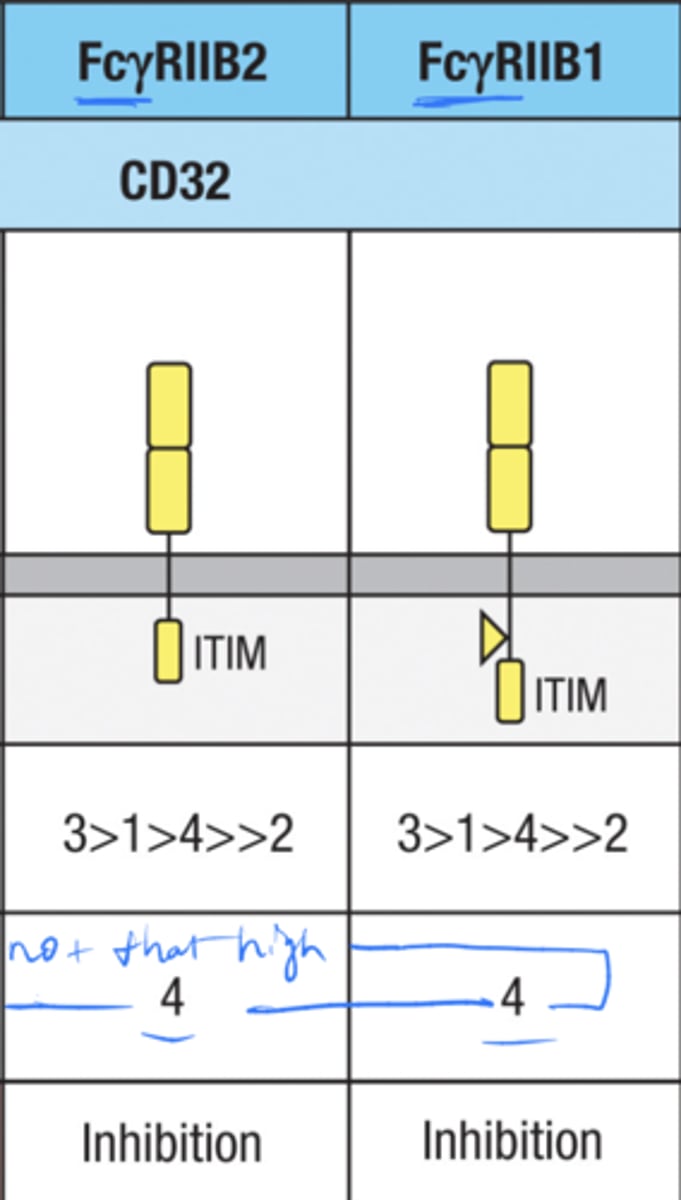
Where is the inhibitory receptor FcgRIIB2 expressed? (5)
Macrophages, neutrophils, and eosinophils, B cells, and mast cells
What is the role of FcgRIIB2 on these cells?
Controls their inflammatory response by antagonizing the actions of activating Fc receptors
The inhibitory receptor FcgRIIB2 is also expressed on
mast cells and B cells.
what are the 3 activating Fc receptors
FcgRI
FcgRIIA
FcgRIII
An Fc receptor acts as an
antigen receptor for NK cells
NK cell can recognize and kill cells that are coated with IgG by the mechanism of
antibody-dependent cell-mediated cytotoxicity (ADCC)
ADCC mechanisms is used to target tumor cells for death -
monoclonal Ab that targets CD20 on B cells. NK cells kill B cell tumors, but the side effect is that they also kill healthy B cells
what is the 4 step process by which Fc receptor acts as an antigen receptor for NK cells
- anti-CD20 binds to CD20 on the surface of B cells
- Fc receptors on NK cells recognize bound anti-CD20 ab
- Cross-linked Fc receptor signal NK cell to kill the B cell
- B cell dies via apoptosis
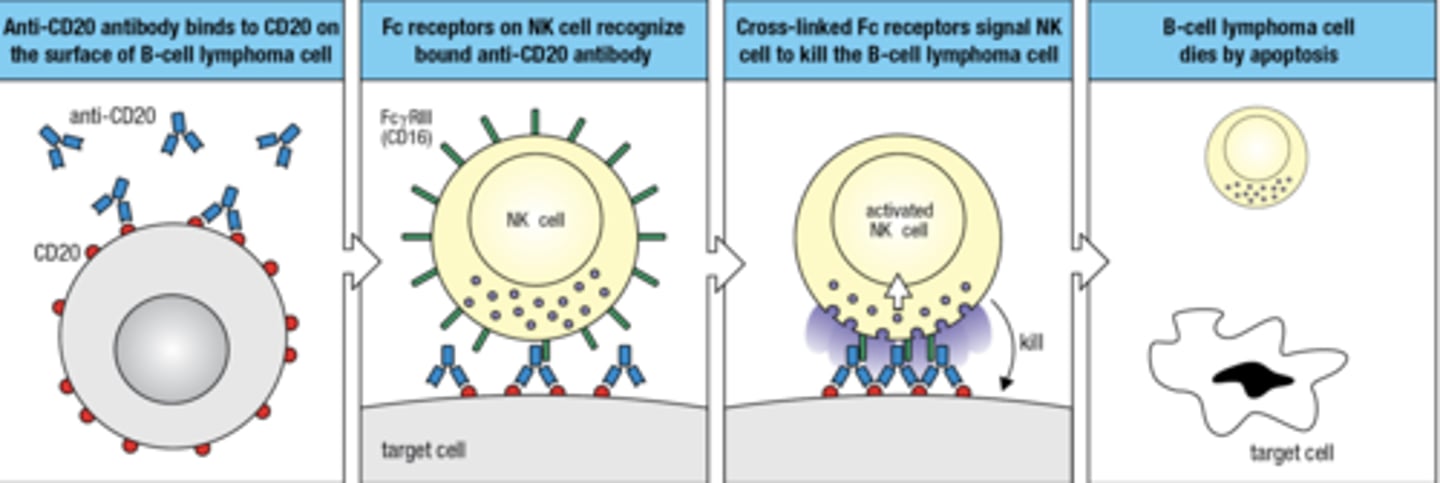
look at this summary slide, love this summary slide
The Fc receptor for monomeric IgA belongs to a different family than the Fc receptors for IgG and IgE

learning outcomes
start now
Compare signal transduction from the B-cell receptor complex with that of the T-cell receptor complex.
Structure and Activation:
B-cell Receptor (BCR):
Composed of a membrane-bound immunoglobulin molecule and a signaling module consisting of the Igα (CD79A) and Igβ (CD79B) proteins.
Antigen binding to the BCR directly triggers receptor aggregation, which activates the signaling cascade.
T-cell Receptor (TCR):
Composed of a variable αβ or γδ TCR complex associated with CD3 and ζ-chain signaling subunits.
Requires antigen presentation by Major Histocompatibility Complex (MHC) molecules on antigen-presenting cells (APCs). The TCR itself does not directly recognize free antigens.
Signal Transduction:
BCR:
Upon antigen binding and receptor aggregation, Src family kinases (like Lyn) phosphorylate the immunoreceptor tyrosine-based activation motifs (ITAMs) on Igα and Igβ.
This leads to the recruitment and activation of Syk, a tyrosine kinase, which further propagates the signal through multiple pathways including phospholipase Cγ (PLCγ), leading to calcium mobilization and activation of protein kinase C (PKC).
These events result in the activation of transcription factors like NF-κB and NFAT that drive B-cell proliferation, differentiation, and antibody production.
TCR:
Binding of the TCR to peptide-MHC complex on APCs leads to phosphorylation of ITAMs on CD3 and ζ-chains by Lck and Fyn, Src family tyrosine kinases.
This recruits ZAP-70, a Syk family kinase, which further phosphorylates downstream adapters and enzymes including LAT and SLP-76.
This results in the activation of several pathways including PLCγ, leading to similar downstream effects as BCR signaling such as calcium influx, activation of PKC, and transcription factors like NF-κB and NFAT.
Compare signal transduction from the B-cell receptor complex with that of the T-cell receptor complex. continued
Differences in Signal Transduction:
Coreceptor Involvement:
TCR signaling often involves coreceptors CD4 or CD8, which bind to MHC class II or class I molecules respectively, enhancing the sensitivity and specificity of TCR-peptide-MHC interactions. These coreceptors also have ITAMs and contribute to the signaling cascade.
BCR does not typically involve such coreceptors in its basic signaling process, though other receptors like CD19 can modulate BCR signal strength.
Antigen Recognition:
TCR recognizes processed peptides presented by MHC molecules, which is a more indirect method of antigen recognition requiring antigen processing and presentation.
BCR can bind directly to native antigens in their native conformations.
Sensitivity
TCR signaling is highly sensitive and tightly regulated, partly due to the need for simultaneous recognition of both MHC and antigen peptide.
BCR signaling can be triggered by cross-linking of receptors through repetitive antigen epitopes, which may lead to a broader range of antigen recognition.
Describe how B cells enter the secondary lymphoid organs, move to the follicles, and interact with follicular dendritic cells that have captured antigen.
1. Entry into Secondary Lymphoid Organs:
B cells enter secondary lymphoid organs, such as lymph nodes, the spleen, or Peyer's patches, primarily through high endothelial venules (HEVs). The entry process is guided by:
Chemokines and Adhesion Molecules: B cells express specific homing receptors such as L-selectin (CD62L), which binds to addressins on the HEVs. Chemokine receptors like CCR7 on B cells are attracted to chemokines CCL19 and CCL21, which are expressed by the endothelial cells of HEVs.
Transmigration: After adhesion, B cells undergo transmigration across the endothelium into the lymphoid tissue. This is facilitated by integrins such as LFA-1 and VLA-4 on B cells binding to their ligands, ICAM-1 and VCAM-1, on the endothelium.
2. Migration to Follicles:
Once inside the lymphoid organ, B cells migrate towards the follicles, which are rich in FDCs and are the primary sites for B cell antigen recognition and subsequent proliferation:
Guidance by Chemokine Gradients: The migration is directed by the gradient of the chemokine CXCL13, produced by FDCs. B cells expressing the receptor CXCR5 follow this gradient to localize within the follicles.
Describe how B cells enter the secondary lymphoid organs, move to the follicles, and interact with follicular dendritic cells that have captured antigen. part 2
3. Interaction with Follicular Dendritic Cells:
In the follicles, B cells encounter FDCs, which play a crucial role in antigen presentation and B cell activation:
Antigen Capture and Display: FDCs capture antigens through their complement receptors (CR1, CR2) and Fc receptors. These antigens are not processed but are presented in their native form on the surface of FDCs. This presentation is crucial for the activation of B cells that recognize specific antigens.
Antigen Recognition: When a B cell's receptor (BCR) binds to its specific antigen displayed on an FDC, it receives a primary signal necessary for activation.
Formation of Immune Synapses: This interaction leads to the formation of an immune synapse where signaling molecules are concentrated, enhancing the B cell activation process.
Co-stimulatory Signals: Additional signals are often required for full activation, which can be provided by other cells (e.g., T helper cells) through molecules such as CD40L.
Describe how B cells enter the secondary lymphoid organs, move to the follicles, and interact with follicular dendritic cells that have captured antigen. part 3
4. B Cell Activation and Response:
Once activated by binding to the antigen and receiving necessary secondary signals, B cells undergo several changes:
Proliferation and Differentiation: Activated B cells proliferate and differentiate into plasma cells that produce antibodies specific to the antigen, or into memory B cells that provide long-term immunity.
Germinal Center Reaction: Some of the activated B cells form germinal centers within the follicles where they undergo somatic hypermutation and class-switch recombination, further refining the antibody response.
5. Exit and Circulation:
Finally, the effector cells (plasma cells) and some memory B cells exit the lymphoid organ to circulate in the bloodstream, reaching various sites in the body to perform their immune functions or to reside until reactivated by the same antigen.
This sequence of events ensures that B cells effectively recognize antigens, become activated, and mount a tailored immune response, crucial for fighting infections and maintaining immunological memory.
Outline the role of T follicular helper cells in inducing both the proliferation and differentiation of antigen-activated B cells.
1. Differentiation and Migration to Germinal Centers:
Differentiation: T_FH cells differentiate from naïve CD4^+ T cells upon activation by antigen-presenting cells (APCs), typically dendritic cells, in the presence of specific cytokines such as IL-6 and IL-21.
Expression of Key Molecules: They express the chemokine receptor CXCR5, which directs their migration towards B cell follicles in response to CXCL13, a chemokine abundantly expressed in these areas.
Localization: T_FH cells localize to the B cell zones of lymphoid follicles where they come into close contact with antigen-specific B cells.
2. Interaction with Antigen-Activated B Cells:
Antigen Presentation: Antigen-activated B cells internalize their antigen, process it, and present peptide-MHC class II complexes on their surface.
Synapse Formation: T_FH cells recognize these peptide-MHC class II complexes through their T cell receptor (TCR). This recognition is stabilized by co-stimulatory interactions, particularly the CD40 ligand (CD40L) on T_FH cells binding to CD40 on B cells.
3. Provision of Help to B Cells:
Cytokine Secretion: T_FH cells secrete a variety of cytokines including IL-21, IL-4, and IFN-γ, which are crucial for B cell proliferation, survival, and differentiation.IL-21: Particularly vital for B cell proliferation and the germinal center response; promotes the differentiation of B cells into plasma cells and memory B cells.IL-4: Supports immunoglobulin class switching to IgG and IgE.IFN-γ: Can influence class switching to IgG2a in mice.
CD40-CD40L Interaction: Provides essential signals that prevent apoptosis of B cells and promote their proliferation and maturation. This interaction also enhances isotype switching and affinity maturation.
Outline the role of T follicular helper cells in inducing both the proliferation and differentiation of antigen-activated B cells. part 2
4. Role in Germinal Center Formation and Maintenance:
Germinal Center Reaction: Within germinal centers, T_FH cells help maintain a selective environment where B cells with higher affinity receptors are favored for survival and expansion.
Affinity Maturation: T_FH cells are involved in the selection process during somatic hypermutation, aiding in the selection of B cells that produce high-affinity antibodies.
B Cell Differentiation: Influence the differentiation pathway of B cells, deciding between memory B cell formation and differentiation into plasma cells, which secrete antibodies.
5. Long-Term Immunity and Memory:
Memory B Cells: T_FH cells also help in the generation of memory B cells, which are crucial for rapid and robust responses upon re-exposure to the same antigen.
Regulation of Response: Their functions are finely regulated to avoid excessive immune reactions. Regulatory mechanisms involve PD-1/PD-L1 interactions which can limit T_FH cell activity.
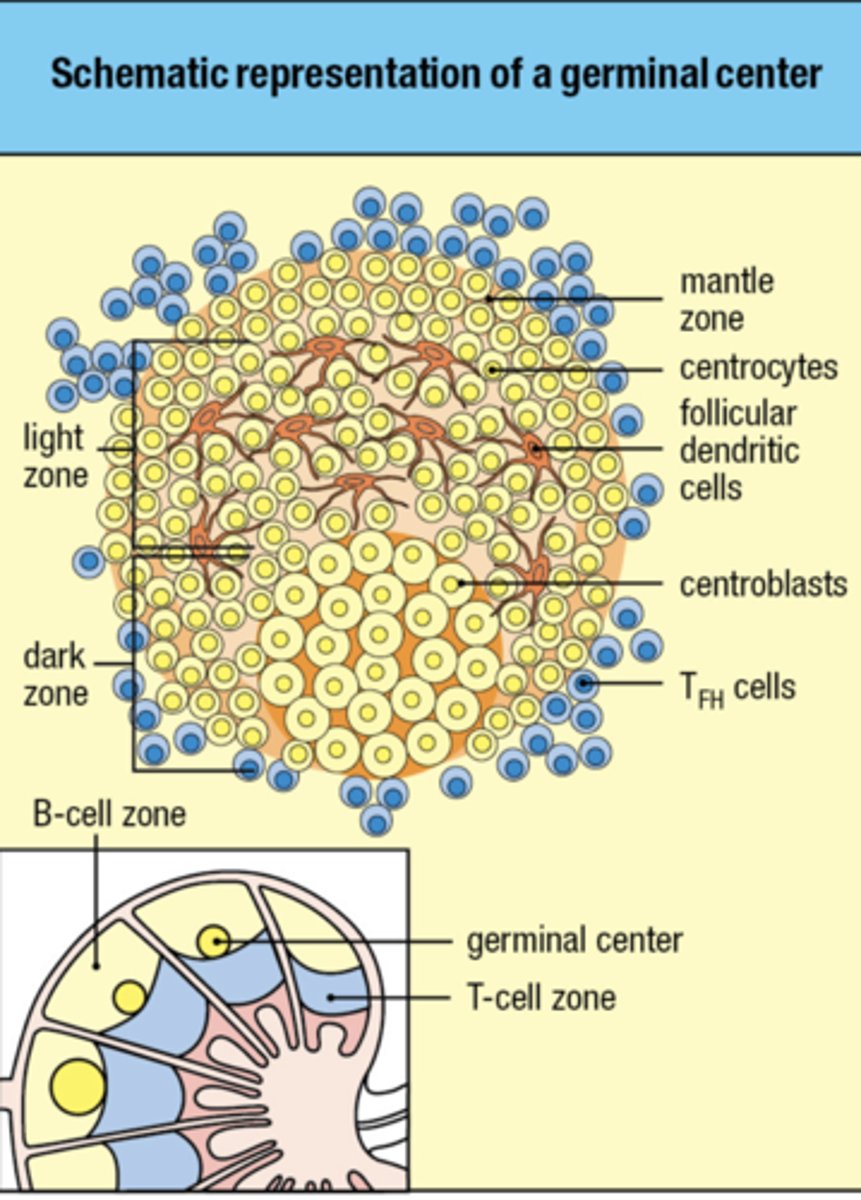
Describe the development and organization of the germinal center, which is the site of affinity maturation and differentiation into plasma cells or memory cells.
Formation and Structure of the Germinal Center:
Initiation:
Antigen Encounter: B cells encounter and bind to their specific antigen, which they process and present on MHC class II molecules.
T Cell Help: Concurrently, T follicular helper (T_FH) cells recognize antigenic peptides presented by B cells. This interaction, particularly through CD40-CD40L binding, is crucial for the initiation of the germinal center response.
Cytokine Influence: Cytokines such as IL-21 and IL-4 secreted by T_FH cells promote the proliferation of antigen-activated B cells and their migration into the follicular dendritic cell-rich areas of lymphoid tissues.
Organization:
Dark Zone (DZ) and Light Zone (LZ) Formation: Once formed, germinal centers are divided into two main areas:Dark Zone: Characterized by densely packed proliferating B cells (centroblasts). Here, B cells rapidly divide and undergo somatic hypermutation in their B cell receptor (BCR) genes, which alters the affinity of the antibodies they produce.Light Zone: Contains fewer, less densely packed B cells (centrocytes), follicular dendritic cells (FDCs), and T_FH cells. Centrocytes present their newly mutated BCRs for antigen trapped on FDCs and receive survival and differentiation signals from T_FH cells.
Describe the development and organization of the germinal center, which is the site of affinity maturation and differentiation into plasma cells or memory cells. part 2
Dynamics within the Germinal Center:
Recycling and Affinity Maturation:
Selection in the Light Zone: B cells (centrocytes) that have acquired mutations leading to higher affinity BCRs are selected for survival. This selection is facilitated by their ability to capture and present antigen more effectively, receiving positive survival signals from T_FH cells.
Recycling: Selected centrocytes may recycle back to the dark zone to undergo further rounds of proliferation and somatic hypermutation, enhancing antibody affinity through iterative cycles.
Differentiation:
Plasma Cell Differentiation: High-affinity centrocytes can differentiate into plasma cells under the influence of cytokines like IL-10. Plasma cells exit the germinal center and migrate to the bone marrow or inflamed tissues to secrete large quantities of high-affinity antibodies.
Memory B Cell Formation: Some of the selected high-affinity centrocytes differentiate into memory B cells, which remain in lymphoid tissues or circulate in the blood, providing long-term immunity.
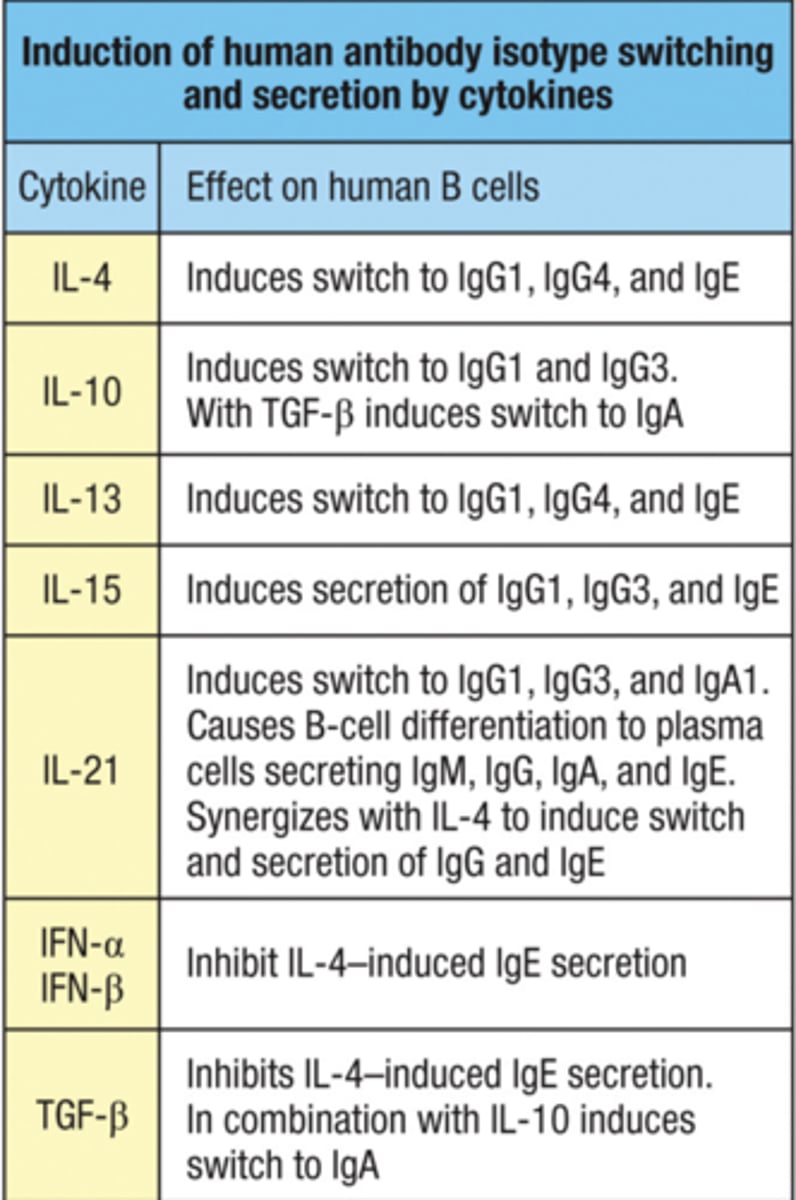
Explain the role of cytokines and CD40L in isotype switching.
Role of CD40L in Isotype Switching:
CD40-CD40L Interaction:
Activation: CD40 is a costimulatory protein found on the surface of B cells, and CD40L (CD154) is expressed on activated T cells, particularly T follicular helper (T_FH) cells.
B Cell Stimulation: The interaction between CD40 on B cells and CD40L on T_FH cells provides essential signals that are critical for B cell proliferation, survival, differentiation, and importantly, for initiating the isotype switching process.
Upregulation of Activation-Induced Cytidine Deaminase (AID):
Enzymatic Role: AID is an enzyme that is vital for CSR. It deaminates cytosine bases in DNA, converting them to uracil, which triggers the molecular mechanisms necessary for isotype switching.
Induction by CD40: The CD40-CD40L interaction significantly enhances the expression of AID in B cells, which is a prerequisite for the initiation of CSR.
Role of Cytokines in Isotype Switching:
Cytokines are signaling molecules secreted by various immune cells, including T_FH cells, and they profoundly influence the choice of antibody isotype to which a B cell switches. Each cytokine can promote a different type of class switch, depending on the nature of the immune response required:
Interleukin-4 (IL-4):
Promotes Switching to IgG1 and IgE: IL-4 is crucial for class switching to IgG1 in response to parasitic infections and to IgE in allergic responses. It acts by activating transcription factors that enhance germline transcription of these isotype genes.
Interferon-gamma (IFN-γ):
Promotes Switching to IgG2a (in mice) and IgG2 (in humans): IFN-γ is produced in response to viral and intracellular bacterial infections, facilitating isotype switching that optimizes macrophage activation and other cellular immune functions.
Explain the role of cytokines and CD40L in isotype switching. part 2
Transforming Growth Factor-beta (TGF-β):
Promotes Switching to IgA: TGF-β plays a critical role in mucosal immunity by inducing class switching to IgA. IgA is essential for neutralizing pathogens at mucosal surfaces.
Interleukin-10 (IL-10):
Promotes Switching to IgG3 and IgA: IL-10 can influence switching to IgG3 in humans, which is important for certain types of bacterial infections, and also supports IgA production alongside TGF-β.
Integration in Immune Responses:
Context-Dependent Regulation: The environment and nature of the antigen challenge dictate which cytokines are produced by helper T cells and other immune cells. This cytokine milieu, in turn, influences the isotype switching in B cells, tailoring antibody responses to be most effective against specific pathogens.
Synergistic Actions: Often, multiple signals including those from cytokines and CD40-CD40L interactions are required simultaneously for effective CSR. This ensures that isotype switching is tightly regulated and occurs in response to appropriate immune challenges.
Connect the isotypes of antibody to their effector functions and the effective clearance of pathogens in distinct anatomical sites.
IgM
Primary Response: IgM is the first antibody produced in response to an infection, prior to isotype switching.
Pentameric Structure: This structure allows IgM to bind effectively to antigens with repeating epitopes, such as bacterial polysaccharides.
Effector Functions: IgM is excellent at activating the complement system, which leads to the lysis of pathogens and facilitates phagocytosis.
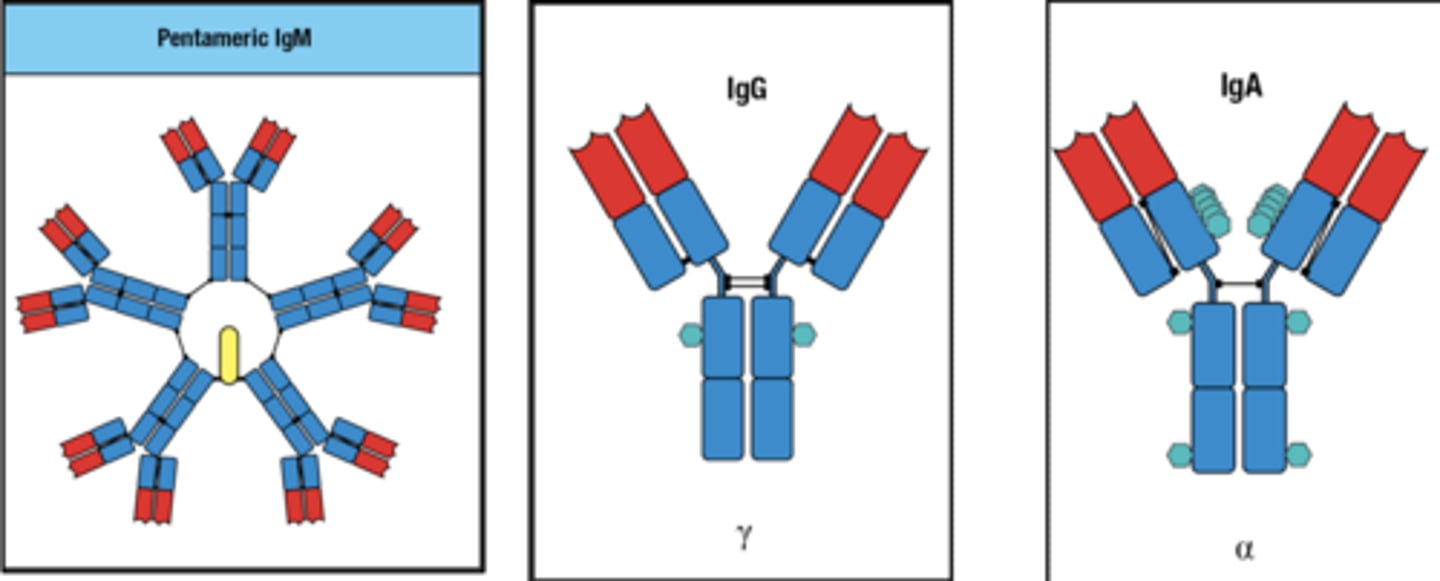
IgG
Most Abundant in Serum: IgG is the most prevalent antibody in blood and extracellular fluid, making it crucial for systemic immunity.
Subclasses: Humans have four subclasses (IgG1, IgG2, IgG3, and IgG4) each adapted to different pathogens and functions.IgG1 and IgG3: Highly effective at activating complement and binding to Fc receptors on phagocytic cells, making them key players in defending against viruses and bacteria.IgG2: More specialized in response to polysaccharide antigens, such as those from encapsulated bacteria.IgG4: Less effective in complement activation but plays a role in immune regulation and response to chronic allergen exposure.
Transplacental Transfer: IgG is the only isotype that can cross the placenta, providing neonatal protection.
IgA
Main Antibody in Mucosal Areas: Predominantly found in the gastrointestinal, respiratory, and urogenital tracts.
Dimeric Structure: In its secretory form, IgA is dimeric, which makes it effective at neutralizing pathogens and preventing their attachment to epithelial cells in mucosal surfaces.
Protective Functions: Prevents colonization by pathogens and neutralizes toxins and viruses at mucosal surfaces without provoking inflammation.
Connect the isotypes of antibody to their effector functions and the effective clearance of pathogens in distinct anatomical sites. part 2
IgE
Role in Allergy and Parasitic Infections: Best known for its role in allergic reactions, IgE is also crucial for defense against helminthic (worm) infections.
Binding to Mast Cells and Basophils: IgE binds to Fc receptors on mast cells and basophils. Upon antigen binding, these cells release histamine and other mediators that contribute to inflammation and expulsion of parasites.
IgD
Less Understood: IgD's role is less well understood compared to other isotypes.
Surface Antibody on B Cells: Primarily found on the surface of B cells as a receptor in its monomeric form and plays a role in initiating B cell activation.
Respiratory Tract: Recent studies suggest that IgD is involved in immune surveillance of the respiratory tract's mucosal surfaces.
IgM and IgG are crucial in the bloodstream and tissue spaces, providing the first line of defense during systemic infections and a sustained response after class switching, respectively.
IgA functions at mucosal surfaces where it prevents infection by blocking the attachment of pathogens to host cells and neutralizing toxins.
IgE plays a vital role in the skin and mucosal surfaces, particularly in combating parasitic infections and in allergic responses.
IgD might play a role in the mucosal immunity of the respiratory tract, though its functions are not as clearly defined as other isotypes.
Explain how antibodies neutralize viruses, bacteria, and bacterial toxins.
Neutralization of Viruses
Blocking Attachment: Antibodies can bind to viral surface proteins that are used to attach to host cells. For example, antibodies targeting the hemagglutinin protein of influenza viruses can prevent the virus from binding to host cell receptors.
Prevention of Viral Entry: Even if a virus attaches to a host cell, antibodies can sterically hinder the conformational changes or fusion events necessary for the virus to enter the cell. For instance, antibodies against the HIV gp120 protein can block the virus from entering T cells.
Agglutination: Antibodies can bind to multiple virus particles, linking them together in a process called agglutination. This aggregation makes the viruses more easily recognized and phagocytosed by immune cells.
Activation of Complement System: Certain classes of antibodies (e.g., IgM, IgG1, IgG3) can activate the complement system, leading to the formation of the membrane attack complex that can lyse some viruses directly or opsonize them for phagocytosis.
Explain how antibodies neutralize viruses, bacteria, and bacterial toxins. part 2
Neutralization of Bacteria
Opsonization: Antibodies coat bacteria, enhancing their uptake and destruction by phagocytic cells such as macrophages and neutrophils. This process is facilitated by the Fc receptors on phagocytes that recognize the Fc region of bound antibodies.
Complement Activation: Similar to viruses, antibody binding to bacteria can activate the complement cascade, resulting in the formation of the membrane attack complex that can directly lyse bacterial cells or mark them for enhanced phagocytosis.
Blocking Adhesion: Antibodies can prevent bacteria from adhering to host cells, a crucial step in many bacterial infections. For example, antibodies against adhesins on the surface of uropathogenic E. coli can prevent urinary tract infections by inhibiting bacterial attachment to the bladder wall.
Neutralization of Motility and Invasion: Antibodies can bind to bacterial components critical for motility (like flagella) or tissue invasion (like invasins), impairing the bacteria's ability to spread within the host.
Neutralization of Bacterial Toxins
Binding to Toxins: Antibodies can bind specifically to toxins released by bacteria, thereby neutralizing their harmful effects. This binding can block the interaction of the toxin with its cellular target, preventing the toxin from exerting its pathogenic effect.
Prevention of Toxin Entry: Some toxins need to enter host cells to cause damage. Antibodies can prevent the entry of these toxins by blocking receptor-mediated uptake or by steric hindrance of the toxin's active sites.
Facilitation of Toxin Clearance: By binding to toxins, antibodies also promote their clearance from the circulation through mechanisms like phagocytosis and degradation in the liver and spleen.
Describe how activating Fc receptors contribute to an effective response through the recruitment of multiple effector-cell types and how inhibitory Fc receptors regulate inflammation.
Activating Fc Receptors
Types and Distribution:
Activating Fc receptors include FcγRI (CD64), FcγRIIA (CD32A), FcγRIII (CD16), and others found on different immune cells such as macrophages, neutrophils, natural killer (NK) cells, and dendritic cells.
Each receptor type has a slightly different affinity for various IgG subclasses, influencing their binding and activation profiles.
Mechanisms of Action:
Phagocytosis: On phagocytic cells like macrophages and neutrophils, Fc receptors recognize and bind to the Fc regions of antibodies that have opsonized pathogens, leading to the engulfment and destruction of these targets.
Antibody-Dependent Cellular Cytotoxicity (ADCC): NK cells use FcγRIII to recognize and kill antibody-coated cells. Upon engagement, these receptors signal the NK cells to release cytotoxic substances that induce apoptosis in the target cell.
Cytokine and Chemokine Release: Binding of antibodies to activating Fc receptors on dendritic cells and other immune cells triggers the release of cytokines and chemokines, which enhance the inflammatory response and recruit additional immune cells to the site of infection.
Activation of Complement System: Some activating Fc receptors can also interact with components of the complement system, further enhancing the clearance of pathogens.
Recruitment of Multiple Effector-Cell Types:
The ability of different cell types to express distinct sets of activating Fc receptors allows for a coordinated and multifaceted immune response. For instance, macrophages can directly phagocytose pathogens, while NK cells can target and eliminate infected cells, providing a comprehensive defense mechanism against a variety of pathogens.
Describe how activating Fc receptors contribute to an effective response through the recruitment of multiple effector-cell types and how inhibitory Fc receptors regulate inflammation. part 2
Inhibitory Fc Receptors
Primary Type - FcγRIIB:
FcγRIIB is the principal inhibitory Fc receptor, expressed on many of the same cells as the activating receptors, including B cells, dendritic cells, and macrophages.
Mechanisms of Action:
Inhibition of Cell Activation: FcγRIIB has an immunoreceptor tyrosine-based inhibition motif (ITIM) in its cytoplasmic domain. When co-crosslinked with an activating receptor (like BCR or activating FcγRs), it recruits phosphatases such as SHIP1 and SHP1, which dephosphorylate key molecules in activating signaling cascades, thereby inhibiting cell activation.
Regulation of Antibody Production: On B cells, FcγRIIB can modulate the activation of the cell following engagement of the B cell receptor (BCR) with antigen, thus playing a role in regulating antibody production and preventing overactivation.
Regulation of Inflammation:
Control of Autoimmunity and Inflammation: By dampening excessive immune cell activation, FcγRIIB helps to prevent autoimmune reactions and controls inflammation. This inhibitory feedback is essential for resolving immune responses and restoring homeostasis after an infection or inflammatory response.
Balance Between Activation and Inhibition: The relative expression levels and signaling balance between activating and inhibitory Fc receptors on cells determine the outcome of receptor engagement, which can vary from potent activation to suppression of an immune response.