Chapter 9 Chem Quiz
1/55
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
56 Terms
Bohr model and the quantum-mechanical model
propose explanations for the inertness of helium, the reactivity of hydrogen, and the periodic law
They explain how electrons exist in atoms and how those electrons affect the chemical and physical properties of elements
Niels Bohr and Erwin Schrödinger (along with albert einstein)
played a role in the development of quantum mechanics, yet bewildered by their own theory of wave-particle duality for the electron
Light
a form of electromagnetic radiation that travels through space at constant speed of 3.0 × 108 m/s (c) (186,000 mi/s)
Modern Atomic Structure
c= λv E= hv E = hc/λ
c= 3.0 × 108 m/s
h= 6.6262x 10-34 Js
Wavelength
λ (lambda); the distance between adjacent wave crests
Spectrum of color
White light contains spectrum of wavelength → spectrum of color
ROYGBIV–seen in a rainbow or when light passes through a prism
Red, orange, yellow, green, blue, indigo, violet
Red light–longest wavelength
Violet light–shortest wavelength
Color seen when it is reflected and all other colors are absorbed
Frequency
v nu; the number of cycles or crests that pass through a stationary point in one second
Relationship between wavelength and frequency
inverse proportion (shorter the wavelength, the higher the frequency and vice versa)
Photon
particle of light; single packet of light energy
Amount of energy depends on wavelength of light–the shorter the wavelength, the greater the energy
Light waves have more energy when their crest are closer together–higher frequency and shorter wavelength
The Electromagnetic Spectrum
Gamma rays
shortest wavelength and more energetic
Produced by the sun, stars, and unstable atomic nuclei on Earth
Excessive human exposure can be dangerous because the high energy of the photons can damage biological molecules
X-rays
pass through many substances that block visible light and are used to image internal bones and organs
Carry enough energy to damage biological molecules
Several yearly exposures are pretty harmless, excessive exposure to x-rays increase cancer risk
Ultraviolet or UV light
component of sunlight that produces sunburn or suntan
Though not as strong as gamma or x rays, has enough energy to damage biological molecules
Increase risk to skin cancer, cataracts, and causes premature wrinkles
Visible light
ranges from violet to red
Do NOT damage biological molecules
Cause molecules in our eyes to rearrange, which sends a signal to our brains that results in vision
Infrared light
felt when hand placed hear a hot object
Warm abjects (even humans) emit infrared light
Invisible to our eyes, sensors for it can detect it and used for night vision technology
Warm objects like humans glow as much as a lightbulb in the visible region of the spectrum
Microwaves
used for radar and in microwave ovens
Efficiently absorbed by water and can heat substances that contain water
Substances that contain water (food) are warmed by radiation of a microwave, but substances that don't have water, like a plate, cannot
Radio waves
longest wavelength
Used to transmit signal used by AM and FM radio, cellular telephones, TV, and other forms of communication
Emission Spectra
White light spectrum is continuous with radiation emitted at every wavelength; the emission spectrum of an individual element includes only certain specific wavelengths
Bohr model
can explain the emission spectrum of hydrogen; each orbit specified by a quantum number (n) which also specifies the orbits energy; cannot predict spectra for atoms with more than one electron
Energy of each Bohr orbit
specified by quantum number n= 1,2,3 is fixed (quantized); like steps of a ladder, each specific distance from nucleus and each at a specific energy
Impossible for an electron to be between orbits in Bohr model
Excitation and Emission
when a hydrogen atom absorbs a quantum of energy, an electron is excited to a higher energy orbit, then the electron relaxes back down to a lower energy orbit emitting a photon of light
Since amt of energy in a photon is directly related to its wavelength, the photon has a specific wavelength
Light emitted by excited atoms consists of specific lines at specific wavelengths, each corresponding to a specific transition between two orbits
Ex: the line at 486 nm in the hydrogen emission spectrum corresponds to an electron relaxing from the n = 4 orbit to the n = 2 orbit
Ex: line at 657 nm (longer wavelength and lower energy) corresponds to an electron relaxing from the n = 3 orbit to the n = 2 orbit
How was the Bohr model successful? What did it fail to do?
It was successful because it predicted the lines of the hydrogen emission spectrum.
It failed to predict the emission spectra of other elements that contained more than one electron.
Quantum mechanical or wave mechanical model
describes electron orbitals, which are electron probability maps that show the relative probability of finding an electron in various places surrounding the atomic nucleus
replaced the Bohr model in the early twentieth century
quantum-mechanical orbitals replaces Bohr obits.
can predict the bright-line spectra of other elements
electron configuration
indicates which orbitals are occupied for a particular atom; shows the occupation of orbitals by electrons for a particular atom
Orbitals
represent probability maps that show a statistical distribution of where the electron is likely to be found
electrons do not behave like particles flying through space
does NOT represent the exact path that an electron takes as it travels through space
Principle quantum number (n)
specify an orbital (or orbitals) and the principal shell of the orbital; lowest-energy orbital in the quantum-mechanical model is called the 1s orbital
The higher the principal quantum number, the higher the energy of the orbital
Possible numbers are n=1, 2, 3 with energy increasing as n increases
Ground state
lowest energy state
Excited state
when an electron is in the higher energy orbital after the absorption of energy by a hydrogen atom that causes the electron to jump (or make a transition) to a higher energy orbital.
All atoms have one ground state and many excited states
Subshell
indicated by the letter, specifies shape; s, p, d, f
s—hold up to 2 electrons in total (1 pair)
p—hold up to 6 electrons in total (3 pairs)
d—hold up to 10 electrons total (5 pairs)
f—hold up to 14 electrons total (7 pairs)
Dot density
proportional to the probability of finding the electron; for the 1s orbital is greatest near the nucleus and decreases farther away from the nucleus–most likely electron found close to the nucleus than far away from it
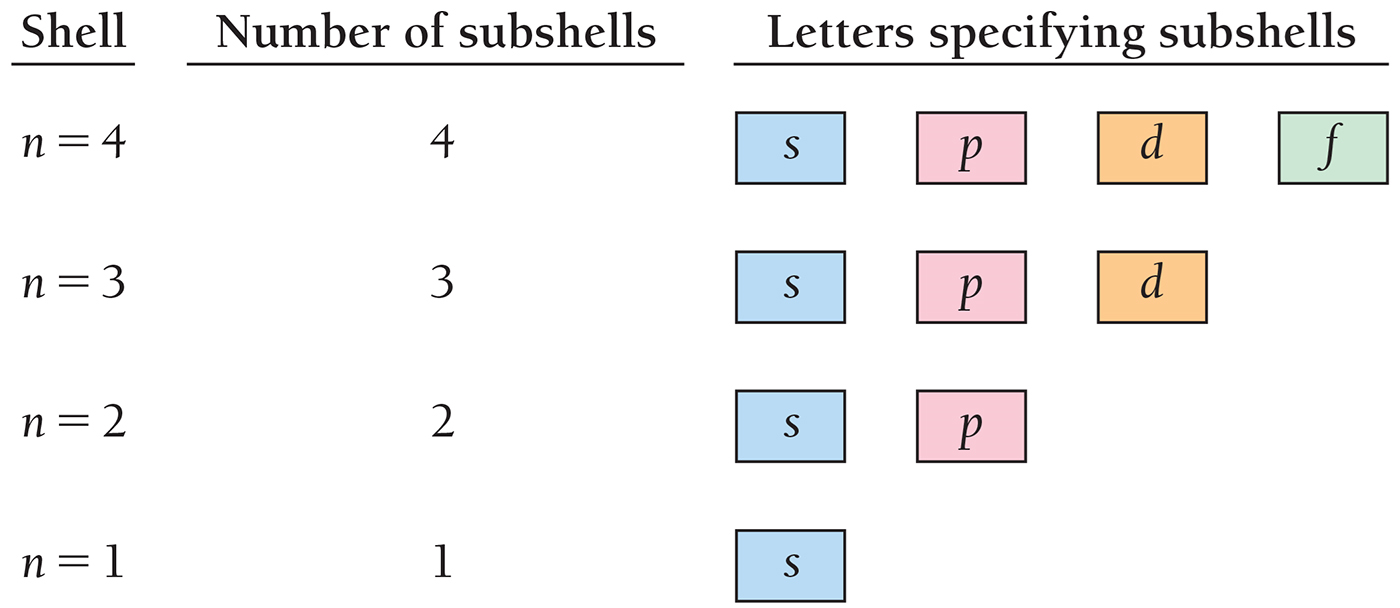
The Number of Subshells in a Given Principal Shell Is Equal to the Value of n
Electron Configurations in the Periodic Table
1s and 2s orbital shape
2p orbital
3d orbitals
Orbital diagram
shows the electrons as arrows in a box representing the orbital
Arrow represents electron spin
Pauli exclusion principle
orbitals may hold no more than two electrons with opposing spins; When two electrons occupy the same orbital, they must have opposing spins
Energy of ordering of orbitals for multielectron atoms
Aufbau Principle
Electrons enter orbitals of lower energy first
The various orbitals within a sublevel have equal energy
The “s” sublevel is always the lowest energy
Hund’s rule
When filling orbitals of equal energy, electrons fill them singly first, with parallel spins
Noble Gas Core Notation
When writing electron configurations for elements beyond neon—or beyond any other noble gas—the electron configuration of the previous noble gas can be abbreviated by the symbol for the noble gas in brackets
Ex: Na: 1s22s22p63s1 → Na: [Ne]3s1
Valence Electrons
are the electrons in the outermost principal shell (the principal shell with the highest principal quantum number, n)
Involved in chemical bonding
Core electrons
Electrons that are not in the outermost principal shell
Periodic trends in Electron Configurations (main group elements)
Number of valence electrons is equal to the group number of its column (besides Helium)
Row number is equal to the number of the highest principal shell
Periodic trends in Electron Configurations (transition metals)
The principal quantum number of the d orbital is equal to the row number minus 1
For the first transition series the outer configuration is 4s23dx (x = number of d electrons)
Two exceptions: Cr is 4s13d5 and Cu is 4s13d10
The number of outer shell electrons in a transition series does not change as you move across a period
transition series represents the filling of core orbitals and the number of outer shell electrons is mostly constant—either 2 or 1.
(2e–) for 4s23dx
(1e–) for 4s13d5 or 4s13d10
Write the Electron Configuration for Any Element Based on Its Position in the Periodic Table
The inner electron configuration is the electron configuration of the noble gas that immediately precedes that element in the periodic table. Represent the inner configuration with the symbol for the noble gas in brackets.
The outer electrons can be determined from the element’s position within a particular block (s, p, d, or f) in the periodic table. Trace the elements between the preceding noble gas and the element of interest, and assign electrons to the appropriate orbitals.
The highest principal quantum number (highest n value) is equal to the row number of the element in the periodic table.
For any element containing d electrons, the principal quantum number (n value) of the outermost d electrons is equal to the row number of the element minus 1
Noble Gases
Atoms with 8 electrons (2 for helium) are predicted to be low in energy and stable
Chemically stable, relatively inert (non reactive)
Electron configurations close to noble gases are most reactive because they can attain noble gas electron configurations by losing or gaining a small number of electrons
Alkali Metals
Most receive since their outer electron configuration is (ns1) is 1 electron beyond noble gas configuration
If react to lose the electron, they attain a noble gas configuration
Alkaline Earth metals
All have electron configurations ns2 and are 2 electrons beyond a noble gas configuration
Tend to lose 2 electrons, forming 2+ ions and attaining a noble gas configuration
Halogens
All have ns2np5 electron configurations therefore 1 short of a noble gas configuration
Tend to gain 1 electron, forming 1- ions and attaining a noble gas configuration
atomic size
distance from the nucleus to the outermost electron
Periodic trend: Atomic Size
As you move to the right across a period in the periodic table, atomic size decreases
atomic size of an atom is determined by the distance between the outermost electrons and the nucleus
Size of an orbital depends on principle quantum number
Each step across period, number of protons increase → greater pull on electrons from the nucleus causing size to decrease
As you move down a column in the periodic table, atomic size increases
the highest principal quantum number, n, increases
Since size of an orbital increases with increasing principal quantum number, the electrons that occupy the outermost orbitals are farther from the nucleus a you move down a column
Ionization energy
the amount of energy needed to remove an electron from a neutral atom, making it a positively charged ion
Ionization energy Periodic Trend
increases as you move right across a period, and decreases as you move down a column in the periodic table
Metallic Character
ease with which an atom becomes a cation
Metallic Character Periodic Trend
decreases as you move right across period and increases as you move down a column in the periodic table
Metals tend to lose electrons in their chemical reactions, while nonmetals tend to gain electrons.
As you move across a period in the periodic table, ionization energy increases, which means that electrons are less likely to be lost in chemical reactions.
Metallic character decreases as you move to the right across a period and increases as you move down a column in the periodic table.