Chem Study Guide
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
Ideal Gas Law
PV = nRT
Value of “R” in PV=nRT
0.0821
Avogadro’s law
V1/N1=V2/N2
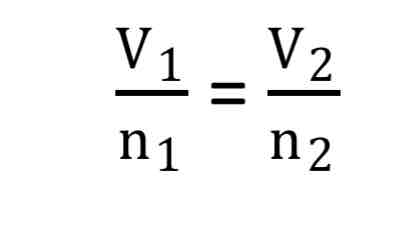
____atm = ____ torr or mmhg
1 atm = 760 torr/mmhg
Boyles Law
P1 x V1 = P2 x V2
Charles Law
V1/T1=V2/T2
Combined Gas Las
(P1 x V1)/T1 = (P2 x V2)/T2
___ celsius = ___ Kelvin
“x” celsius = “x” + 273 kelvin
Troposphere
3/4 of mass of atmosphere are found in this layer
Most weather occurs in this layer
Temperature generally decreases with height due to moving away from the
ground heat
Stratosphere
2nd layer of the atmosphere from the ground
Temperature constant or increasing with height
Ozone layer is found here and it absorbs energy carried in UV
Mesosphere
3rd layer of the atmosphere from the ground
Temperature generally decreases with height due to getting away from ozone heating
Meteors usually get burned in this layer
absolute zero
the lowest temperature at which the motion of particles are minimal. This is 0K or -273°C
Thermosphere
4th layer of the atmosphere from the ground
Temperature generally increases with height due to the absorption of shortest wavelength UV light
Aurora is found in this layer
diffusion
Mixing of different gases
Effusion
A gas escaping through a small opening into an empty container
Elastic collision
No energy is lost from collision. Object bounces off
Inelastic collision
Object loses kinetic energy after collision. Slows down or stops after collision
Components of atmosphere
Nitrogen gas (78.08%)
•Oxygen gas (20.95%)
•Argon gas (0.93%)
•Carbon dioxide gas (0.033%)
Rest made up of small amounts of methane, ammonium, helium, neon, krypton
STP
Standard temperature and pressure
0 degree celsius
1 atm
22.4 L
Kinetic molecular theory
Size of gas particles so small that the size of it can be considered negligible(zero)
Particles do not repel/attract one another
Particles in constant random motion, colliding with walls of container. These collisions result in pressure
Molecules bounce elastically off container, not losing kinetic energy
average kinetic energy is proportional to kelvin temp