Chemistry C9: Crude oil and fuels
1/55
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
56 Terms
Is crude oil a finite resource or infinite?
Finite
Will eventually run out
Where is crude oil found?
In rocks
How is crude oil formed?
Over millions of years from the remains of plankton buried in mud
Layer upon layer of rock was laid down on top creating conditions to make crude oil
Conditions needed to make crude oil
High pressure
High temperature
Absence of oxygen
What is crude oil?
Mixture of hydrocarbons
Remains of an ancient biomass consisting mainly of plankton that was buried in mud
Hydrocarbons
Molecules made up of hydrogen + carbon atoms only
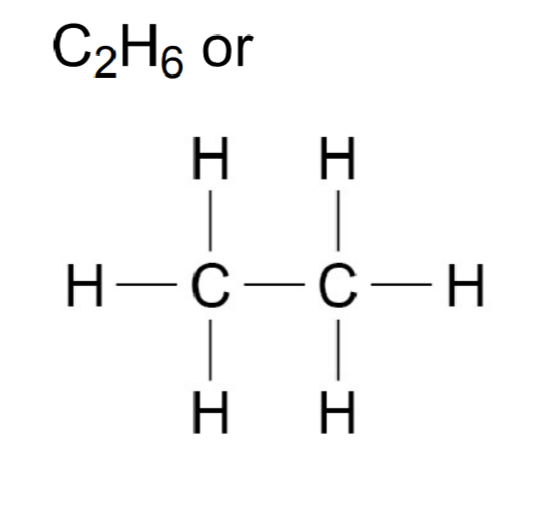
Alkanes
General formula: CnH2n+2
Saturated molecules
Hydrocarbons
Why are alkanes saturated molecules?
Only single covalent bond between C atoms
So contain as many H atoms as possible
C atoms are fully bonded to H atoms
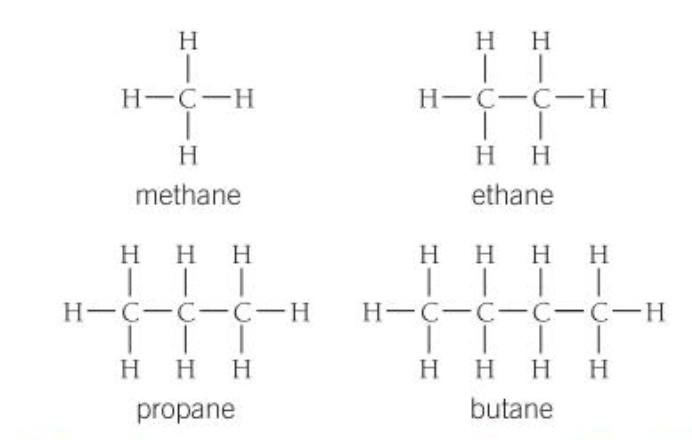
First 4 alkanes
Methane- CH4
Ethane- C2H6
Propane- C3H8
Butane- C4H10
Viscosity
Thickness of a fluid
High: flow slowly
Flammability
How easily a hydrocarbon combusts
Short chain: Very flammable
Boiling point
Temperature at with a liquid changes to a gas
Volatility
Tendency to turn into a gas
Saturated
Single covalent bond between C atoms
What happens to … as the size of a hydrocarbon molecule increases and why?
Viscosity
Flammability
Boiling point
Increases
Decreases
Increases
What does the properties of hydrocarbons depend on?
Size of molecule
ie chain length of their molecules
Why are short-chain hydrocarbon molecules more useful than longer-chain ones?
Lighter fractions = make better fuels
Ignite easily
Burn well
Less smokey flames (cleaner)
How do you make hydrocarbons in crude oil useful?
Separate them
Use fractional distillation
Fractions
Contain hydrocarbons with a similar number of C atoms
In FD, crude oil separated into fractions
Fractional distillation of crude oil
Crude oil heated to v high temp
It boils- all hydrocarbons evaporate + turn into a gas
Crude oil vapours enter the FD column (hotter at bottom, cooler at top)
HC vapours rise up the column
HC condense when they reach their BP
Liquid fractions are then removed
Temperatures in the fractional distillation column
Hotter at bottom
Cooler at top
What happens to very long chain hydrocarbons in fractional distillation?
Have very high BP
So are removed at the bottom of the column
What happens to very short chain hydrocarbons in fractional distillation?
Very low BP
Don’t condense
So are removed from the top of the column as gases
What fractions are used as fuels?
Petrol + diesel: to fuel cars
Kerosene: jet fuel
Heavy fuel oil: to power ships
Liquified petroleum gas: camping stoves
What are some chemicals used as for the petrochemical industry?
Feedstock
Solvents, lubricants, detergents, polymers
Feedstock
A chemical used to make other chemicals
What does the combustion of hydrocarbon fuels do?
Release energy
What happens to the carbon and hydrogen atoms during combustion?
React with oxygen
Oxidised
Products of complete combustion of a hydrocarbon (unlimited O2)
CO2
H2O
How to test for products of complete combustion of a hydrocarbon
CO2, limewater, turns cloudy
Water, blue cobalt chloride paper, turns pink
Water, white anhydrous copper sulfate, turns blue
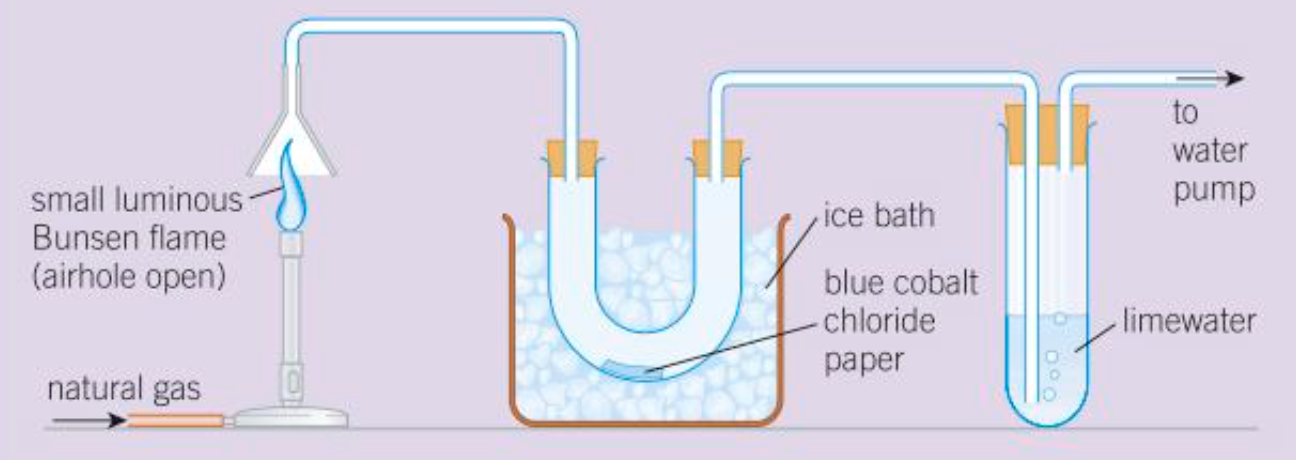
Products of incomplete combustion
carbon monoxide, carbon, water
Why is carbon monoxide dangerous?
Toxic gas
Colorless + odourless
CO binds to RBC instead of O2
Compare complete with incomplete combustion
Products
How much O2 is available
Bunsen flame used
How much energy is released
CC:
CO2 + H2O
Excess O2
Blue flame
Max amount
IC:
CO + H2O
Limited O2
Orange
Limited
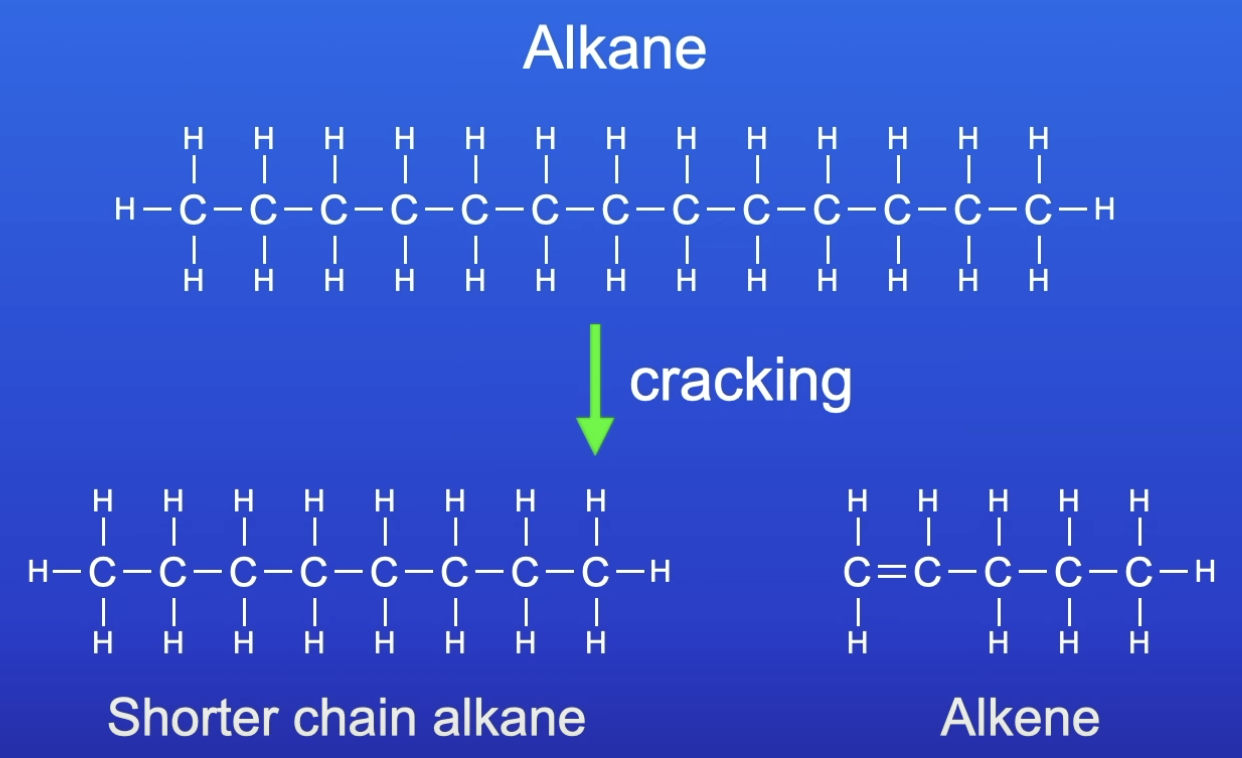
Why are larger, less useful hydrocarbon molecules cracked?
To produce smaller, more useful molecules
LC HC = poor fuels (difficult to vaporise)
Cracking
Long chain alkane is broken down (cracked) to produce smaller chain, more useful ones by thermal decomposition
Main demand for crude oil
Fuels
Feedstock (starting materials) in chemical industry
Products of cracking
Alkane
Alkene
2 methods to carry out cracking
Catalytic cracking
Steam cracking
Conditions catalytic cracking
High temperature
Catalyst (speed up reaction)
Conditions for steam cracking
High temperature
Steam
Process of cracking
A heavy fraction distilled from crude oil is heated to vaporise the hydrocarbons
The vapour is then either:
Passed over a hot catalyst
Mixed with steam + heated to a v high temp
Where does cracking occur in?
Crackers
At an oil refinery in steel vessels
What type of reaction is cracking?
Thermal decomposition
Alkene
CnH2n
Unsaturated HC
Double CB betw 2 C atoms
What are alkenes used for?
To make polymers
As starting materials for other useful chemicals (feedstock)
Which is more reactive: alkanes or alkenes
Alkenes more reactive than alkanes
How can you test for alkenes (unsaturated hydrocarbon)
Use bromine water (orange)
Shake alkene with bromine water
Turns colourless
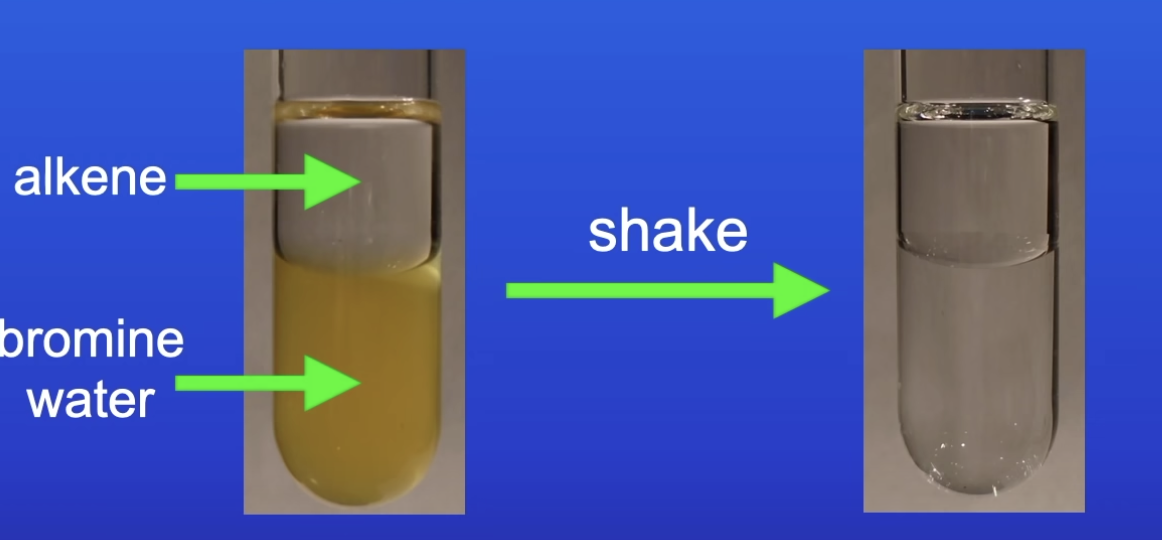
How to distinguish between alkanes and alkenes using bromine water
Alkane + BW = no color change (orange)
Alkene + BW = becomes colorless
Which hydrocarbons are saturated and unsaturated?
Alkanes = saturated
Alkenes = unsaturated
Why do alkenes and alkanes react differently?
Presence of a double covalent bond between 2 C atoms
What are the fractions produced from the FD of crude oil processed to produce?
Fuels
Feedstock for the petrochemical industry
What do the vast array of natural + synthetic carbon compounds occur due to?
The ability of C atoms to form families of similar compounds
Complete combustion of a hydrocarbon fuel
Releases energy
C + H2 in the fuels are oxidised.
Produces CO2 + H2O
What are hydrocarbons cracked (broken down) to produce?
Smaller, more useful molecules
What are some of the products of cracking used for + why?
Fuels
High demand for fuels w small molecules
What does the FD of crude oil do?
Separates the hydrocarbons from crude oil into fractions