chapter 16.2; mass, energy, and the theory of relativity
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
equation of the equivalence between matter and energy
E=mc²
E stands for energy
m stands for mass
mass is a measure of the quantity of matter
c is a constant that relates the 2, the speed of light (3 × 108 m/s)
matter can be converted into energy, and energy can be converted into matter
complete conversion of 1 gram of matter (approximately 1 paperclip) would produce as much energy as the burning of 15,000 barrels of oil
allows us to calculate that the amount of energy radiated by the Sun could be produced by the complete conversion of about 4 million tons of matter into energy inside the Sun each second
antiparticle
for each kind of particle that exists, there is a corresponding antiparticle
if the particle carries a charge, the antiparticle has the opposite charge
positron
antielectron
has the same mass as the electron but is positively charged
antiproton
has a negative charge
antimatter
when a particle comes into contact with its antiparticle, the original particles are annihilated, and substantial amounts of energy in the form of photons are produced
Since our world is made exclusively of ordinary particles of matter, antimatter cannot survive for very long
individual antiparticles are found in cosmic rays (particles that arrive at the top of Earth’s atmosphere from space)
individual antiparticles can be created in particle accelerators
neutrino
type of elementary particle
energy seemed to disappear when certain types of nuclear reactions took place, violating the law of conservation of energy
physicist Wolfgang Pauli suggest that neutrinos were particles with zero mass, and that like photons, they moved with the speed of light
detected in 1956
The reason it was so hard to find is that neutrinos interact very weakly with other matter and therefore are very difficult to detect
most neutrinos can pass completely through a star or planet without being absorbed
3 different types
500000 times smaller than electrons
atomic nucleus
inside, particles are held together by the strong nuclear force
short range force, only capable of acting over distances about the size of an atomic nucleus
an attractive force
if particles come together under the strong nuclear force and unite to form an atomic nucleus, some of the nuclear energy is released
the energy given up in such process is called the binding energy of the nucleus
binding energy of the nucleus
the energy given up/released when particles come together under the strong nuclear force in the nucleus and unite to form an atomic nucleus
the resulting nucleus has slightly less mass than the sum of the masses of the particles that come together to form it
energy comes from the loss of mass, loss of mass is only a small fraction of the mass of 1 proton
binding energy for different atoms
binding energy is greatest for atoms with a mass near that of the iron nucleus (protons + neutrons=56), less for atoms with both heavier and lighter nuclei
therefore Iron is the most stable element, since it gives up the most energy when it forms, it requires the most energy to break it back down to its component particles
nuclear fusion
when light atomic nuclei come together to form a heavier one (up to iron), mass is lost and energy is released
nuclear fission
when energy is produced by breaking up heavy atomic nuclei into lighter ones (down to iron)
sometimes occurs spontaneously in some unstable nuclei through the process of natural radioactivity
how can nuclei come together close enough to participate in fusion?
if we get protons close enough together within ‘striking distance’ of the nuclear force, they will come together with a much stronger attraction
two protons can fuse only in regions with a temperature of over 12 million K, the speed of the protons average around 1000km/s or more
On average, a proton will rebound from other protons in the Sun’s crowded core for about 14 billion years, at the rate of 100 million collisions per second, before it fuses with a second proton
nuclear reactions in the sun’s interior
three step process that occurs inside the Sun that takes 4 hydrogen nuclei and fuses them together to form a single helium nucleus
the helium nucleus is slightly less massive than the four hydrogen nuclei that combine to form it, and that mass is converted into energy
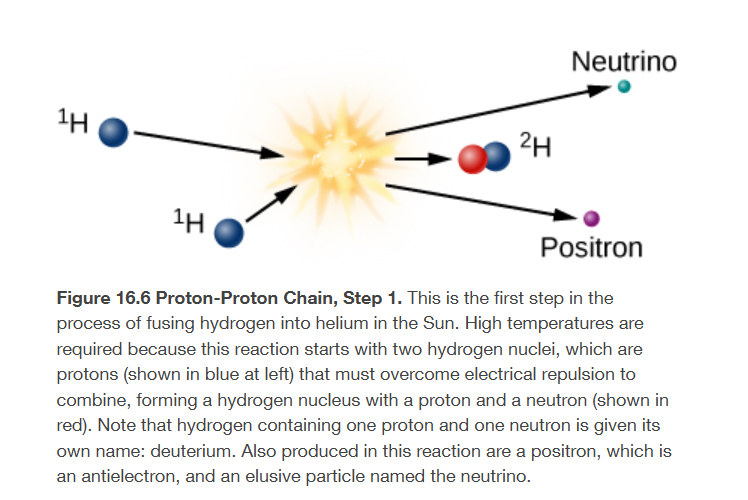
initial step to form a helium nucleus from four hydrogen nuclei
high tempers, two protons combine to make a deuterium nucleus, which is an isotope of hydrogen that contains one proton and one neutron
of the the original protons has been converted into a neutron in the fusion reaction
electric charge has to be conserved in nuclear reactions, and it is conserved in this one
a positron emerges from the reaction and carries away the positive charge originally associated with one of the protons
since its antimatter, the positron will collide with a nearby electron, both will be annihilated, producing electromagnetic energy in the form of gamma-ray protons
this gamma ray collides with particles of matter and transfers its energy to one of them
the particle later releases another gamma-ray photon, but often the mitted photon has a bit less energy than the one that was absorbed
by the time they reach the surface, gamma-ray photons are converted into separate lower-energy photons of sunlight
neutrino also emitted, which travel directly to the Sun’s surface than out into space
move at speed of light, so they escape the Sun about 2 seconds after being created
can take 100,000 - 1,000,000 years for the emission of energy to reach the surface of the Sun from the core
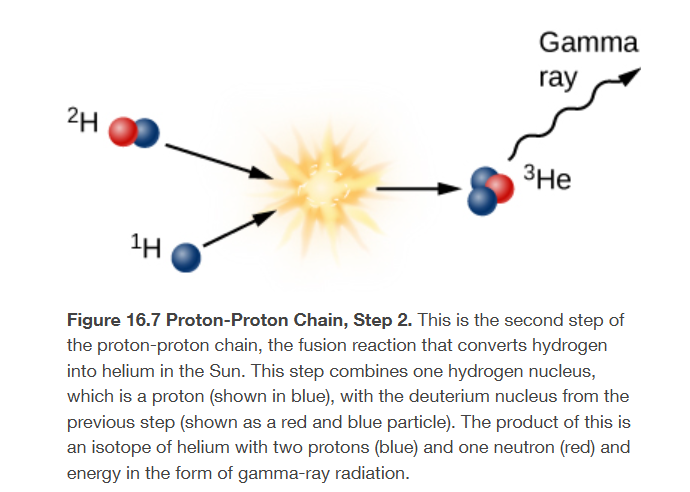
second step to form a helium nucleus from four hydrogen nuclei
add another proton to the deuterium nucleus to create a helium nucleus that contains two protons and one neutron
some mass is lost, more gamma radiated emitted
called helium-3
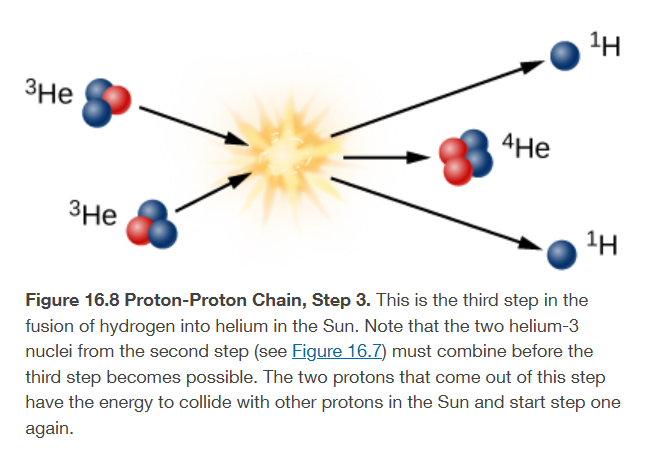
thirds step to form a helium nucleus from four hydrogen nuclei
helium-3 must combine with another helium-3 to form helium-4
two energetic protons left over from this step, which come out of the reaction to collide with other protons to stars the 3 steps again
proton-proton chain
first step: takes a long time
second step: happens after 6 seconds
third step: a million years after
protons collide directly with other protons to form helium nuclei
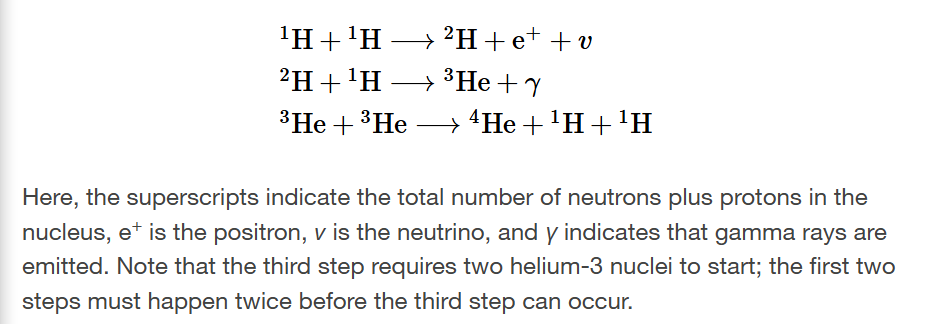
CNO cycle (carbon-nitrogen-oxygen)
occurs in hotter stars than the Sun