20.3 the acid dissociation constant Ka + 20.4 the pH of weak acids
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
why are week acids week
they only partially dissociate
Ka expression
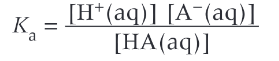
what is pKa
a way of comparing numbers with negative indices
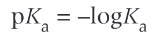
how does the strength of an acid affect the Ka value
strong acid = large Ka value
week acid = small Kc value
how does the strength of an acid affect the pKa value
strong acid = small pKa value
weak acid = large pKa value
2 approximation which simplify the Ka equation in weak acids
the H+ ions from the dissociation of water are very small and can be neglected
neglect and decrease in the concentration of HA from dissociation
simplified Kexpression
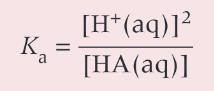
how to calculation [H+] of a week acid given its concentration + Ka
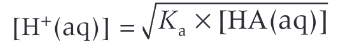
when will the approximations needed to calculate weak acids begin to break down
the first approximation assumes that the dissociation of water is negligible which will break down for very week acids/ dilute solutions
the second approximation assumes that the concentration of acid is much greater than the H+ concentration at equilibrium. this is not justified for stronger weak acids