To measure the relative molecular mass of a volatile liquid (using a conical flask)
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
Theory
• A volatile liquid is a liquid with a low boiling point Example: Propanone: Boiling point =56°C
• Its relative molecular mass (Mr) can be found using experimentation and the ideal gas law
Procedure
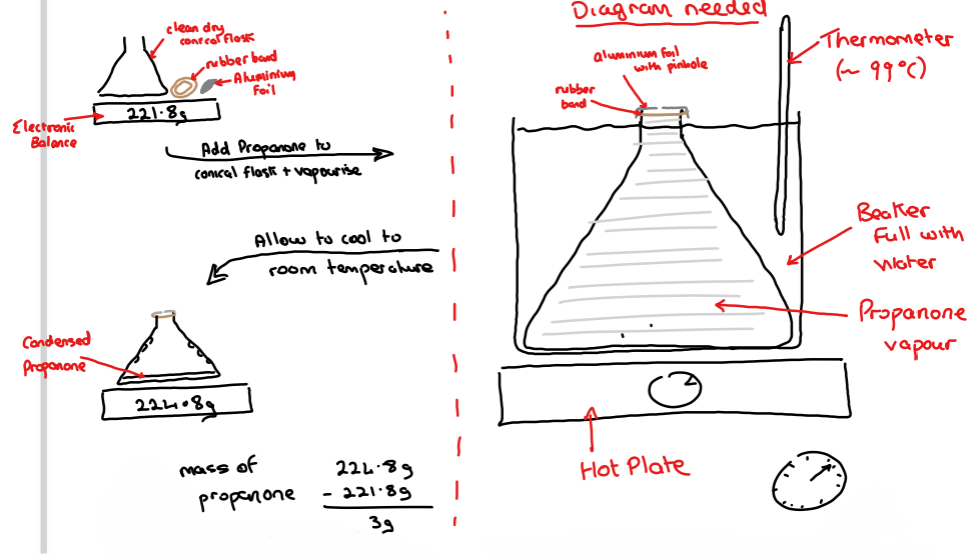
➢ Using an electronic balance, the mass of a clean dry conical flask, a rubber band and a piece of aluminium foil are found
➢ The conical flask is placed into a beaker completely full of water and a small volume ( ̴10cm3) of propanone is added to the conical flask
➢ The opening to the conical flask is covered in the aluminium foil, fastened with the rubber band and a small pinhole is made in the aluminium foil
➢ The water is heated using a hot plate to close to the boiling point of water ( ̴99˚C) meaning the propanone liquid will all vapourise
➢ The conical flask is removed from the boiling water and allowed to cool to room temperature - the vapour will condense, (drops seen on the inside of the conical flask)
➢ The outside of the flask and aluminium foil is dried to remove any water
➢ The mass of the conical flask, rubber band and aluminium foil are found again
➢ Subtracting the initial mass of conical flask, rubber band and aluminium foil away from the final mass of conical flask with vapour, rubber band and aluminium foil will give the mass of the volatile liquid that had vapourised and condensed again
➢ Using the equation of state for an ideal gas PV = nRT, the relative molecular mass (Mr)of the volatile liquid can be calculated
What is meant by a volatile liquid? Name a volatile liquid suitable for this experiment
• A volatile liquid is a liquid with a low boiling point (lower than water)
• Propanone/cylcohexane is a volatile liquid suitable for this experiment
How is the mass of the volatile liquid established?
• Using an electronic balance, the mass of a clean dry conical flask, a rubber band and a piece of aluminium foil found
• After the volatile liquid has been vapourised, allowed to fill the conical flask, removed from the boiling water and allowed to cool and condense again, the outside of the flask and aluminium foil is dried to remove any water
➢ The mass of the conical flask, rubber band and aluminium foil are found again
➢ Subtracting the initial mass of conical flask, rubber band and aluminium foil away from the final mass of conical flask with vapour, rubber band and aluminium foil will give the mass of the volatile liquid that had vapourised and condensed again
How is the volatile liquid vapourised?
• The volatile liquid is placed in a conical flask which is submerged in a beaker completely full of water.
• A hot plate is used to heat the beaker of water close to boiling point – this will vapourise the volatile liquid as its boiling point is below that of water’s
How is the temperature of the vapour obtained?
The temperature of the vapour is obtained by using a thermometer to measure the temperature of the water – the temperature of the vapour can be assumed to be equal to the temperature of the water
How is the pressure of the vapour in the conical flask measured?
• A barometer is used to measure atmospheric pressure.
• The pressure of the vapour in the conical flask will be the same as atmospheric pressure
Explain why the pressure of the vapour in the conical flask is the same as the atmospheric pressure?
The pin hole made in the aluminium foil means the conical flask containing the vapour is in contact with the atmosphere
How is the volume of the propanone vapour found?
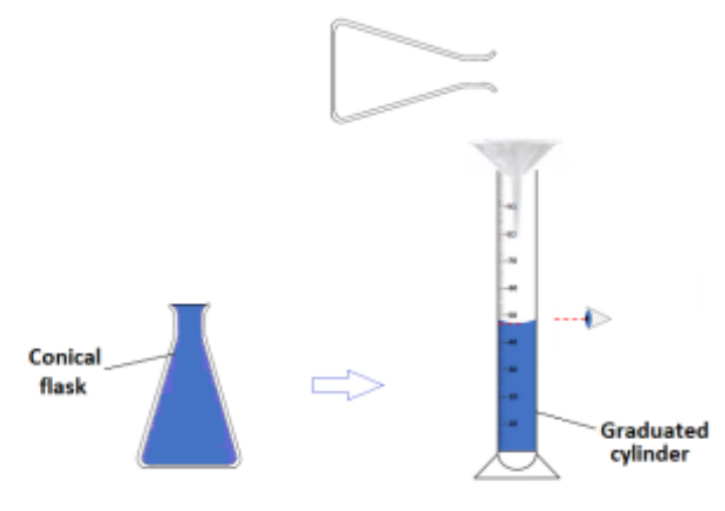
The volume of the vapour is found by filling the conical flask that will be filled with the vapour completely with water, pouring it into a graduated cylinder and reading its volume
Why is this method unsuitable for non-volatile liquids?
The boiling points of non-volatile liquids are too high – boiling water will not vapourise them
What modern instrumental technique can be used as a more accurate method to measure the relative molecular mass of volatile and non-volatile liquids as well as of solid and gaseous substances?
Mass spectrometry
Give three errors in this experiment that may lead to inaccurate results
1. The entire conical flask should be covered in boiling water to ensure the temperature of the vapour is the same as the temperature of the water – this is difficult to achieve
2. The outside of the conical flask and aluminium foil may not be completely dry when finding their mass for the second time
3. The electronic balance may only read to an accuracy of one or two decimal places