9.10.25 Histamine and Antihistamines (Gardner)
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
51 Terms
autocoids (“self remedy”)
naturally occurring substances
local hormones
histamine
serotonin
bradykinin
prostaglandins
eosinophil chemotactic factor
leukotrienes (SRS-A) → slow-reacting substances of anaphylaxis (mixture of leukotrienes)
histamine, β-imidizolethylamine, 2-(4-imidazoyl)ethylamine
fairly ubiquitous
occurs in plants and animals
component of many venoms, toxins, and stinging secretions
1st synthesized in 1907; later isolated from mammalian tissue
histamine hypothesis → hypersensitivity
histamine has a central role in allergic reactions; mediates many classic symptoms of allergic responses → immediate hypersensitivity; primary mediator of type I allergic rxns including anaphylaxis
species and tissue variation in action
histamine
1910 → first detected as uterine stimulant in ergot extracts
1927 → best, dale, dudley, and thorpe → isolated histamine from liver and lung, established as natural constituent
1928 → lewis → provided evidence that “H-substance” with properties of histamine was released from cells of skin by injurious including Ab-ag complex
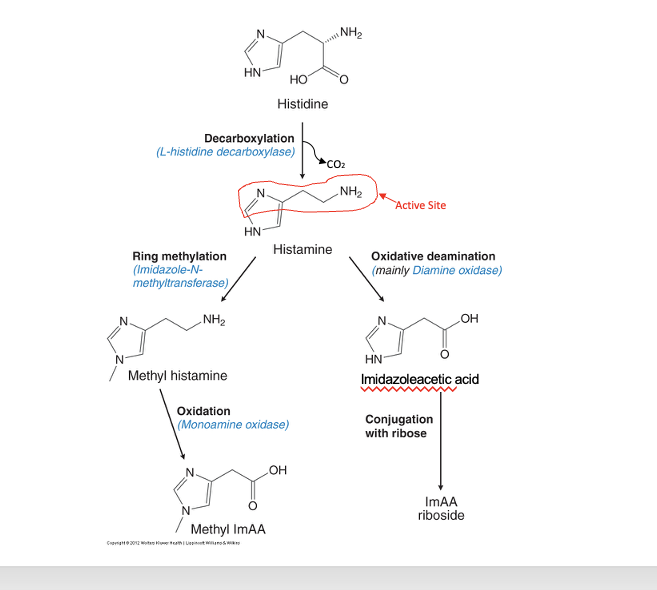
histamine synthesis and inactivation
histidine → histamine
decarboxylation; thru L-histidine decarboxylase
CO2 gets removed
histamine (active group = amine group)
→ methyl histamine
ring methylation; thru imidazole-N-methyltransferase
→ imidazoleactic acid
oxidative deamination; mainly thru diamine oxidase
methyl histamine → methyl ImAA
oxidation; thru monoamine oxidase
imidazoleacetic acid → ImAA riboside
conjugation with ribose
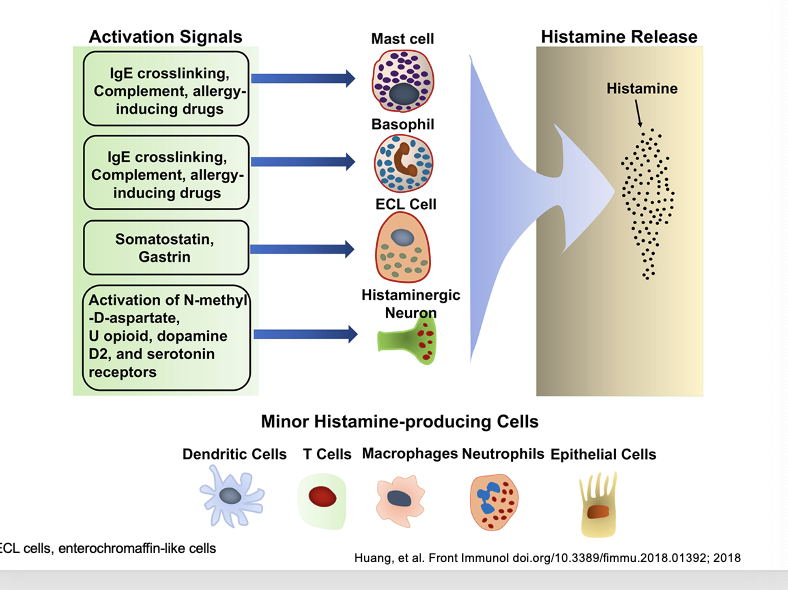
major histamine-producing cells vs minor histamine-producing cells
activation signals → cells → histamine release
IgE crosslinking (allergen binding to IgE antibodies), complement, allergy-inducing drugs → mast cell → histamine release
IgE crosslinking, complement, allergy-inducing drugs → basophil → histamine release
somatostatin, gastrin → ECL cell → histamine release
activation of N-methyl-D-aspartate, U opioid, dopamine D2, and serotonin receptors → histaminergic neuron → histamine release
minor histamine-producing cells
dendritic cells
T cells
macrophages
neutrophils
epithelial cells
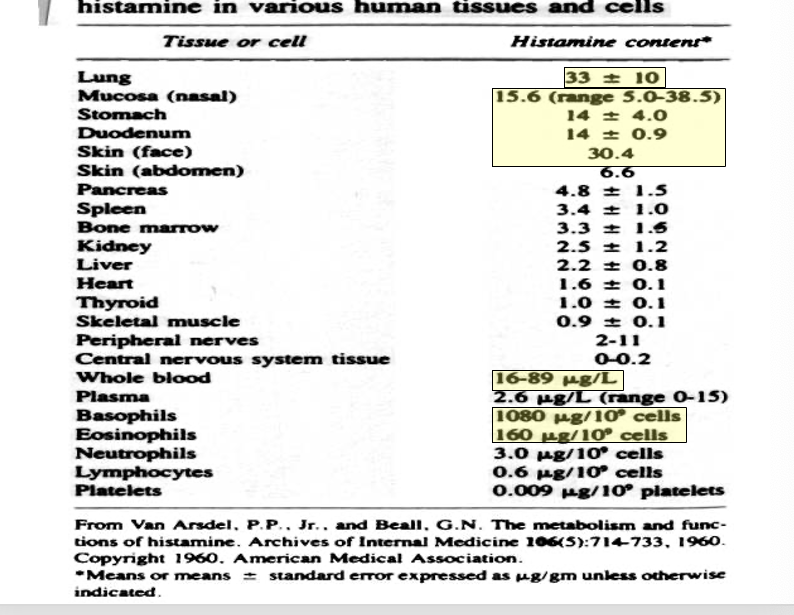
histamine in various human tissues and cells
lung → 33 ± 10 histamine cotent
mucosa (nasal) → 15.6 (range 5.0-38.5)
stomach → 14 ± 4.0
duodenum → 14 ± 0.9
skin (face) → 30.4
whole blood → 16-89 ug/L
basophils → 1080 ug/109 cells
eosinophils → 160 ug/109 cells
histamine distribution and storage
slow turnover rate
mast cells → tissue
basophils → blood
stored in granules
to exert its action, must be released from storage granules
stored in complex with:
heparin
chondroitin sulfate
sulfated polysaccharide
eosinophil chemotactic factor
IL-8
proteases
rapid turnover rate
gastric enterochromaffin-like cells
histaminergic CNS cells
NO storage; produced on demand
regulation
histamine release is controlled by negative feedback which allows for autoregulation
histaminergic neurons → regulation thru muscarinic and H3 receptors
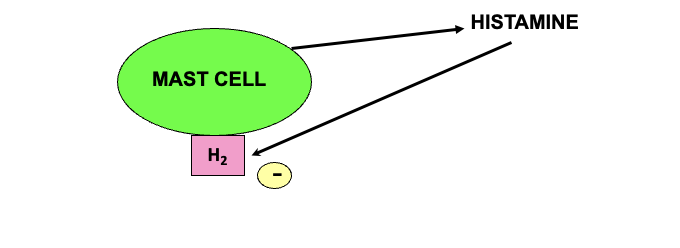
when histamine is released from mast cell it sends a negative signal to H2 receptor → generates an inhibitor signal to reduce further histamine release
histamine releasers
certain drugs
morphine, turbocurarine
these drugs trigger histamine release WITHOUT requiring energy
chemical or mechanical injury
compound 48/80
causes specific degranulation
requires energy and Ca2+
mast cell degranulation stimulated by…
Ca2+ ionophore
conditions that release histamine
tissue injury
allergic reactions → type 1 hypersensitivity rxns
drugs and other foreign compounds
morphine
dextran
antimalarial drugs
dyes
antibiotic bases
alkaloids
amides
quarternary ammonium compounds
enzymes (PLC)
penicillins
tetracyclines
basic drugs
amides
amidines
diamidines
toxins
venoms
proteolytic enzymes
bradykinin
kallidin
substance P
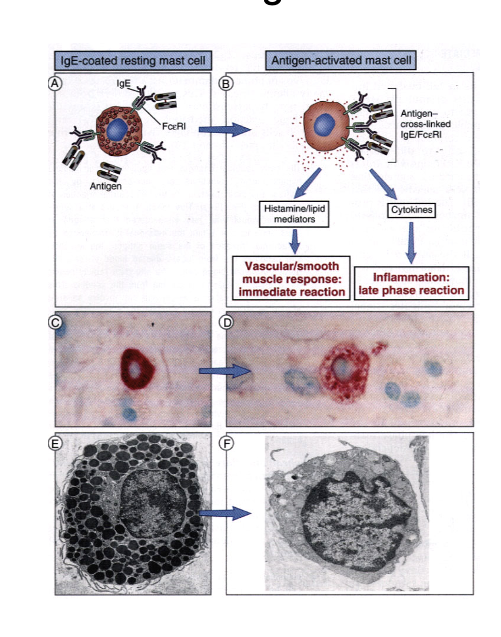
type 1 hypersensitivity and mast cell degranulation
IgE-coated resting mass cell
antigen activated mast cell
→ histamine/lipid mediators released → vascular/smooth muscle response: immediate rxn
→ cytokines released → inflammation: late phase rxn
atropy = sustained, inappropriate IGE responses to common environmental antigens encountered at mucosal surfaces, usually familial association (strong hereditary component)
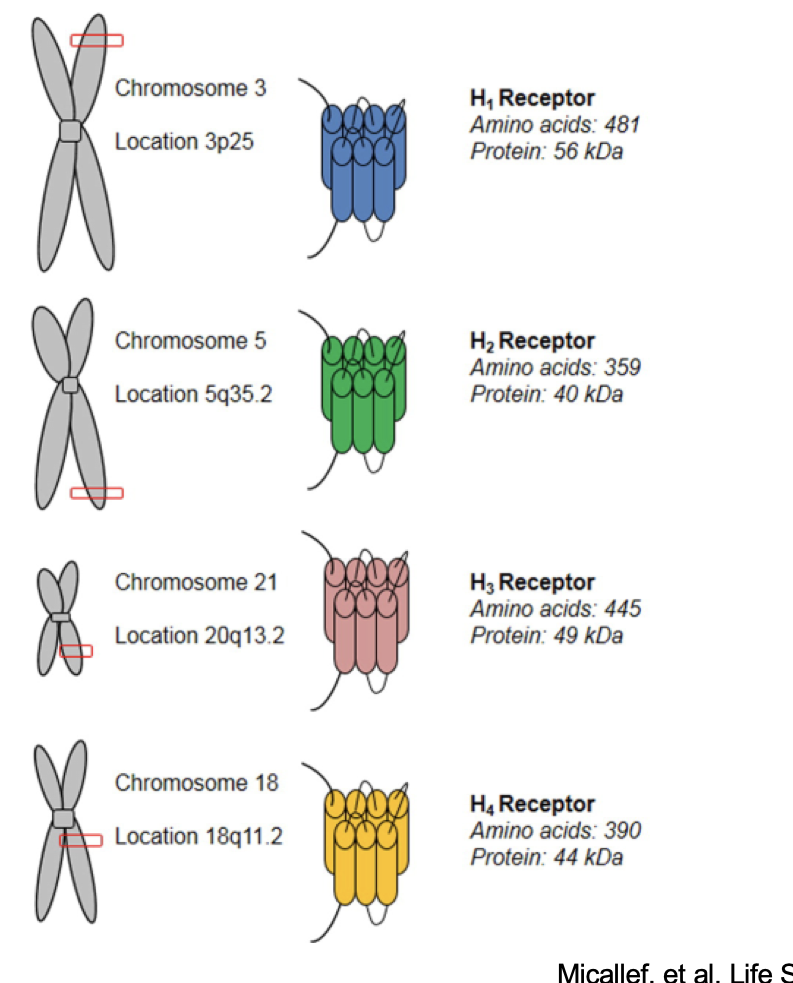
histamine receptors
H1 receptor
on chromosome 3 @ 3p25
H2 receptor
on chromosome 5 @ 5q35.2
H3 receptor
on chromosome 21 @ 20q13.2
H4 receptor
on chromosome 18 @ 18q11.2
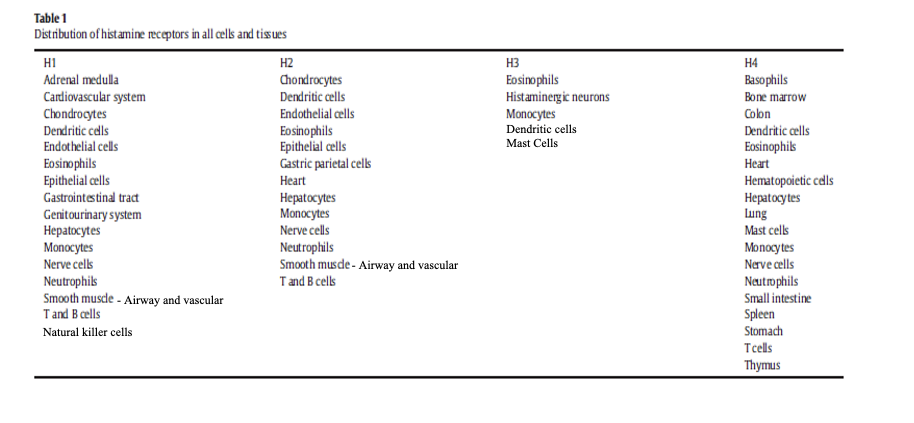
distribution of histamine receptors
H1 found in CV, smooth muscle, immune cells
H2 found in gastric parietal cells, heart, various immune cells
H3 found in histaminergic neurons, some immune cells
H4 found in mast cells, basophils, eosinophils, bone marrow, spleen
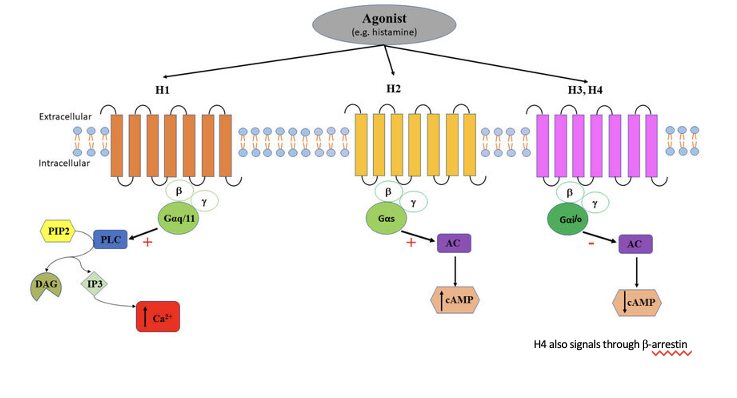
histamine receptor signaling
H1
agonist binds to H1 receptor → activates Gαq/11 → activates PLC → PIP2 cleaves PLC into DAG and IP3 → IP3 increases Ca2+
H2
agonist binds to H2 receptor → activates Gαs → activates AC → increases cAMP
H3/H4
agonist binds to H3/H4 receptor → activates Gαi/o → inhibits AC → decreases cAMP
H4 also signals through β-arrestin
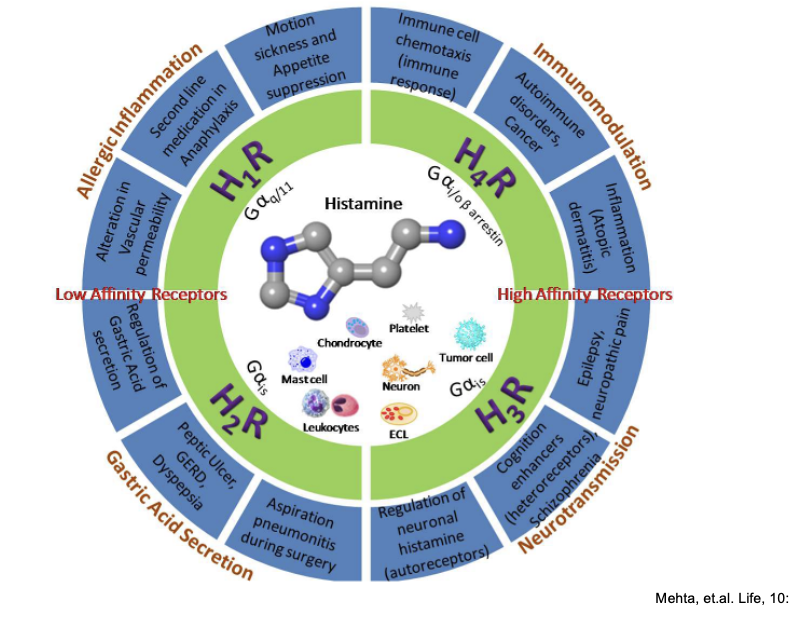
overview of main functions of histamine receptors
low affinity receptors = H1/H2
high affinity receptors = H3/H4
H1
G protein
Gαq/11
function = allergic inflammation
alteration in vascular permeability
targeted for 2nd line medication in anaphylaxis
drugs targeted to this receptor help with
motion sickness and appetite suppression
H2
G protein
Gαis
function = gastric acid secretion
regulation of gastric acid secretion
treatment target for peptic ulcer, GERD, dyspepsia
prevention of aspiration pneumonitis during surgery
H3
G protein
Gαi/o (i think the diagram is wrong)
function = neurotransmission
regulation of neuronal histamine (autoreceptors)
cognition enhancers (heteroreceptors), schziophrenia
epilepsy, neuropathic pain
H4
G protein
Gαi/o
function = immunomodulation
auto-immune disorders (e.g. cancer)
inflammation (associated with atopic dermatitis)
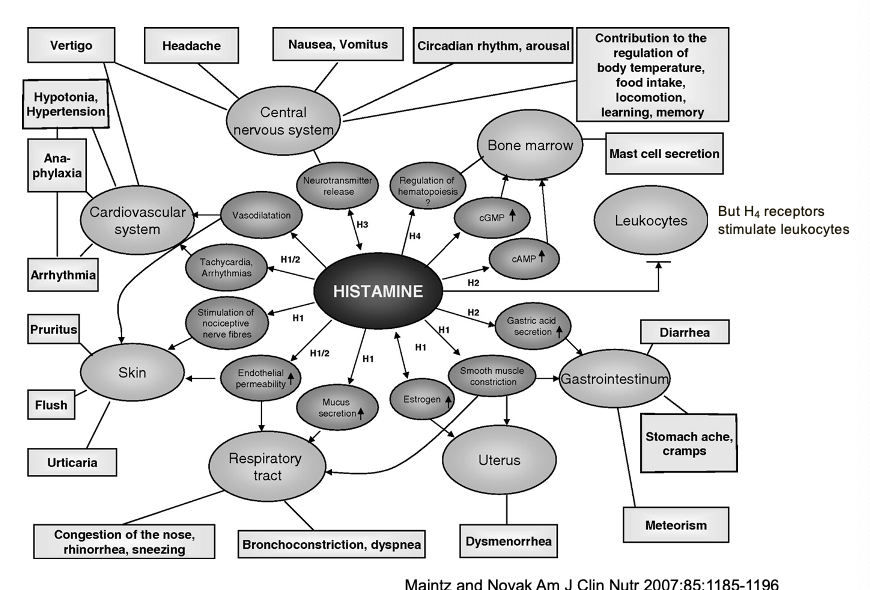
functions of histamine in man
H4 receptors stimulate leukocytes
CNS
primary function = regulation of fundamental brain processes
CV
major effects = vascular and cardiac responses
skin
classic allergic manifestations = vasodilation, sensory effects, inflammatory responses
respiratory tract
airway responses = secretory effects; muscle effects
GI system
digestive effects = secretory function, motility effects
reproductive system
uterine effects = smooth muscle contraction
hematopoietic system
immune cell regulation = bone marrow effects, cGMP/cAMP modulation
pharmacological effects of histamine
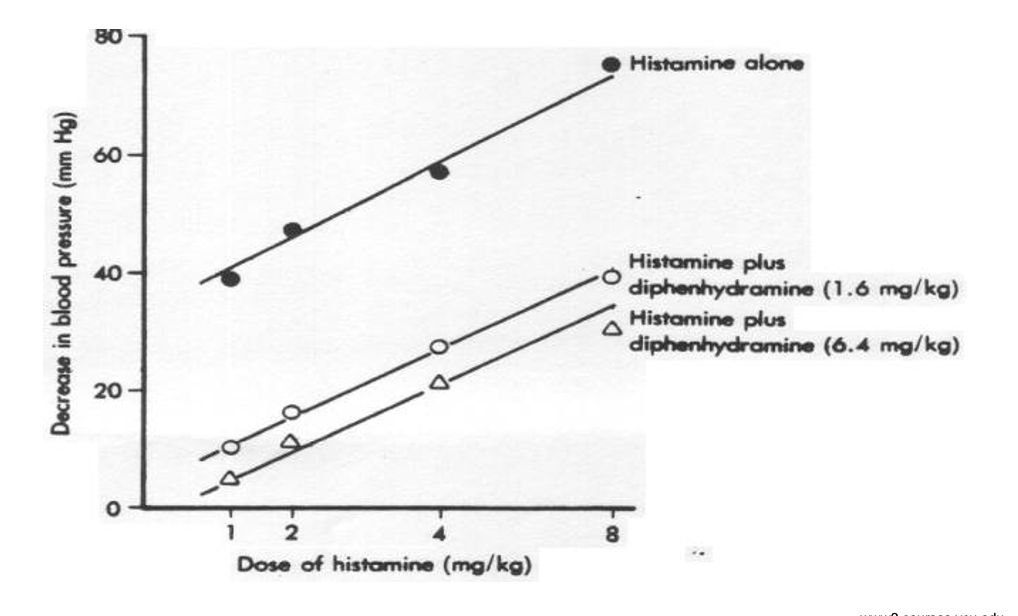
effects of histamine on blood psi
histamine alone greatly decreases blood psi as dose increases
adding diphendryamine (H1) → reduces the fall in blood pressure produced by histamine (dose dependent)
pharmacological effect of histamine pt 2
i want to kms
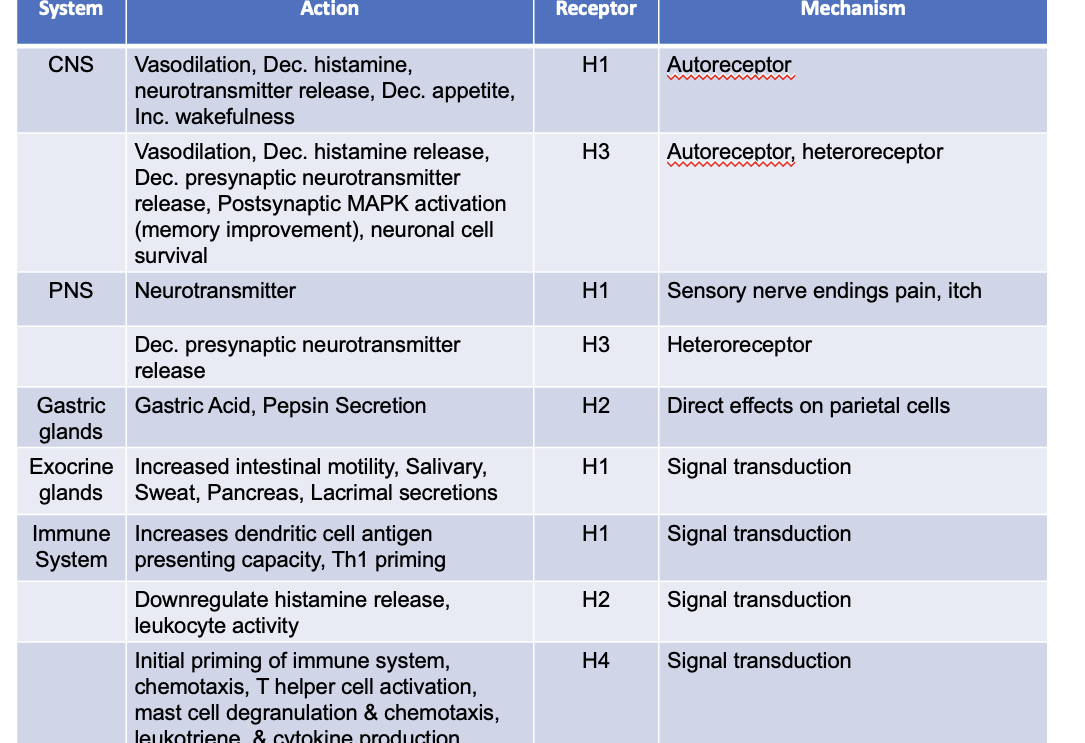
influence of histamine on mucosal associated immune cell subsets
H1R
DC cells express mutliple histamine receptors: H1R, H2R, H4R → activation of H4R on DCs leads to chemotaxis, attracting these cells into the tissue → IL-12 produced by DCs stimulate nearby Th1 that express H1R → enhances IFN-g production and Th1 cell proliferation → H2R antagonizes this effect
on DC cells → increases antigen-presenting capacity and Th1 priming
H2R
on Th2 cells → suppresses IL-4 and Il-13 production and Th2 cell proliferation
on DC cells H2R induces IL-10 production, supresses Ag presentation and supports development of Treg cells
on Treg cells → histamine enhances IL-10 production → H2R potentiate suppressive effect of TGF-β
histamine agonists
small modifications or subsitutions on imidizole ring modify selectively for H1, H2, H3 or H4
2-methylhistamine → selective for H1
4-methylhistamine → H2/H4
R-α-methylhistamine → H3
examples of histamine agonists
betazole (gastramine, histalog)
H2 agonist
diagnostic
testing gastric acid secretion
impromidine
H2 agonist and H3 antagonist
diagnostic
testing gastric acid secretion
clonidine (catapres)
H2 agonist
antihypertensive
clobenpropit
H3 antagonist and H4 agonist
H1 histamine antagonists
physiological antagonists = drugs that counteract the physiological action of histamine
examples: epinephrine; ephedrine
nonspecific
use for
acute anaphylaxis
bronchial asthma
release inhibitors = drugs that prevent histamine release from mast cells
inhibit degranulation of mast cells resulting from IgE
prophylactic (used to prevent a condition before it happens)
ex. cromolyn sodium (gastrocrom)
β2-adrenoreceptor agonists also reduce histamine release
ex. albuterol (ventolin)
H1 antihistamine history
1944 → daniel bovet synthesized first antihistamines
compounds appeared to prevent binding of histamine to H1 receptor thru their structural similarities
H1 inverse agonists
“classical” antihistamine
displaces histamine from H1 receptor
competitive inhibitor
H1 receptor blockade prevents histamine activity and leads to decrease in Ca2+ inside of the cell
acts as inverse agonists → bind and stabilize inactive conformation of H1 receptor
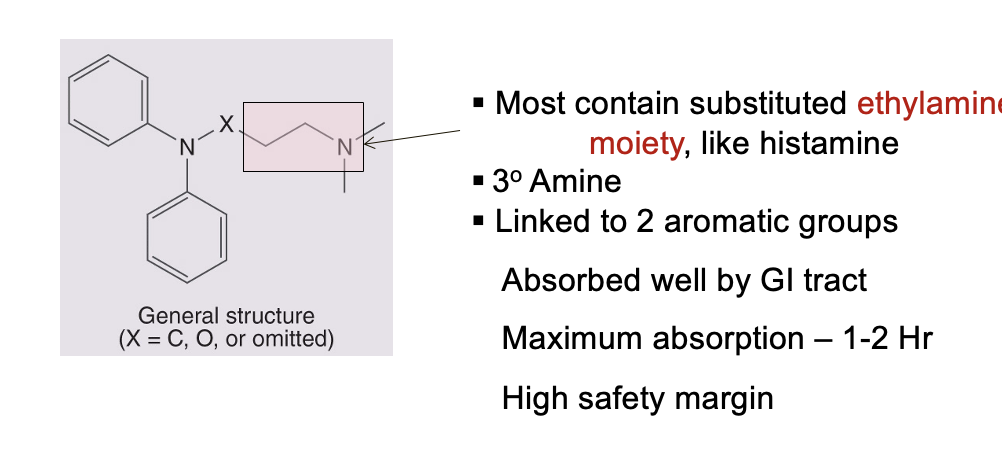
general structure and properties of 1st generation antihistamines
stable, lipid soluble amines
most contain substitued ethylamine moiety similar to histamine
3o amine
linked to 2 aromatic groups
absorbed well by GI tract
maximum absorption = 1-2 hr
high safety margin
similar pharmacological actions
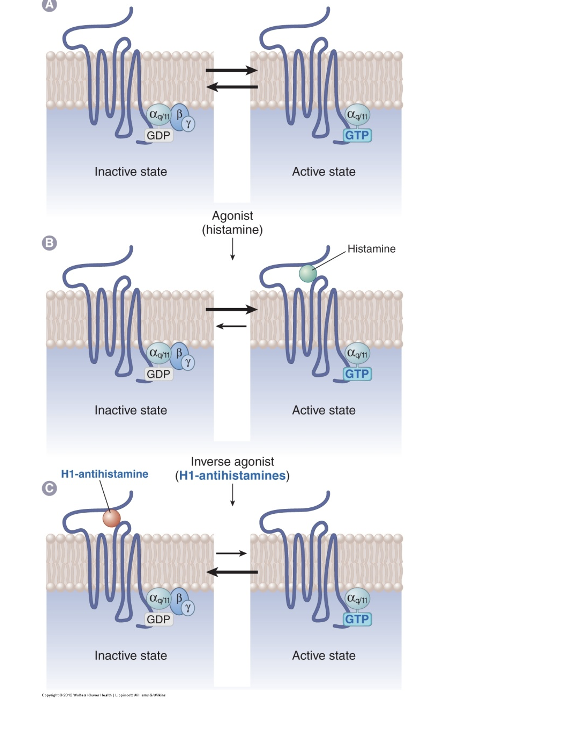
H1 receptor states
panel A: basal receptor activity
inactive state
Gαq/11 = G protein
GDP is bound
active state
GTP is bound
panel B: agonist action (histamine)
inactive state
GDP is bound
active state (when histamine binds to receptor)
GTP is bound
leans more towards active state
panel C: H1-antihistamine (inverse agonist)
inactive state (when H1-antihistamine is bound)
GDP is bound
active state
GTP is bound
lean towards inactive state
pharmacological properties of H1 inverse agonists
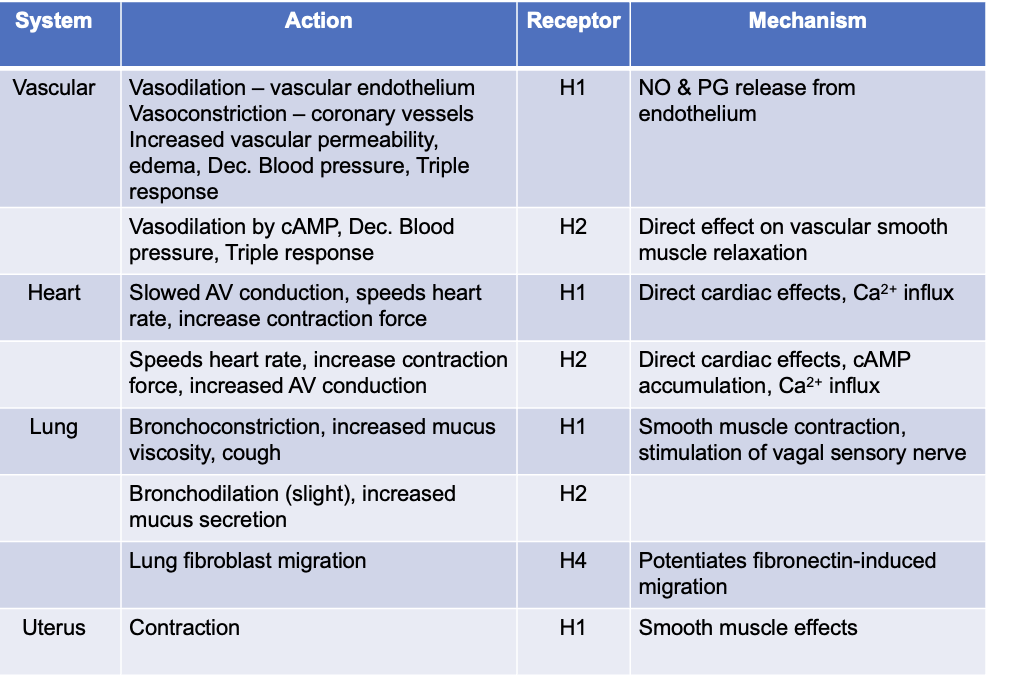
inhibit smooth muscle response to histamine in GI, respiratory tract, small blood vessels (H1)
strongly antagonize increases in capilary permeability (inhibit edema formation, wheal, flare, pain and itching) (H1)
local anesthetic action → due to effects on nerve endings (sensory nerves - reduce pain and itch) (H1)
NO effect on histamine release, gastric acid secretion (H2)
partial effect on vasodilation (blood psi) (H1 and H2)
minimal effect on bronchoconstriction in man; NO effect on H3 or H4 functions
clinical usefulness → mostly allergic conditions involving mucous membranes and skin
actions NOT caused by H1 blockade
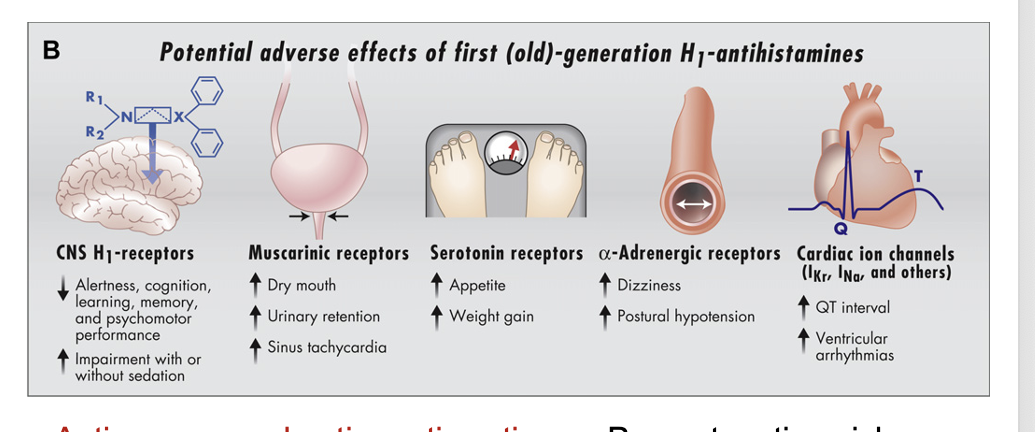
potential adverse effects of 1st generation H1-antihistamines
CNS H1 receptors
decrease alertness, cognition, learning, memory, and psychomotor performance
increase impairment with or without sedation
muscarinic receptors
increase dry mouth
increase urinary retentin
increase sinus tachycardia
sertonin receptors
increase appetite
increase weight gain
α-adrenergic receptors
increase dizziness
increase postural hypotension
cardiac ion channels (IKr, INa, and others)
increase QT interval
increase ventricular arrhythmias
anti-nausea and anti-emetic actions
prevent motion sickness
block histaminergic signal from vestibular nucleus to the medulla
anti-parkinsonism effects
due to anti-cholinergic effect
examples of 1st gen H1 antihistamines
chlorpheniramine (chlortrimeton)
diphenylhydramine (benadryl)
dimenhydrinate (dramamine)
doxylamine (unisom)
2nd generation antihistamines
generally do NOT cause sedation and drying
do NOT cross BBB
lipophobicity
large molecular size
electrostatic charge
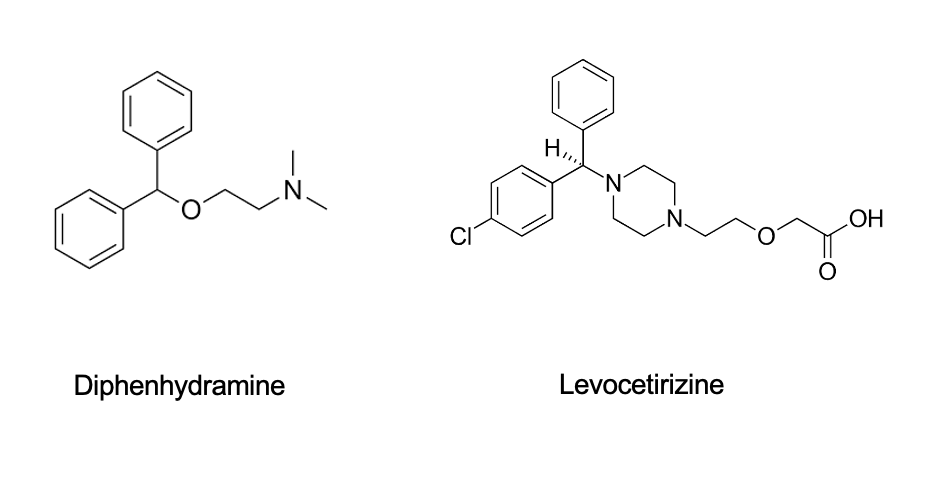
1st vs 2nd gen antihistamines structure comparison
diphenhydramine = 1st gen; levocetirizine = 2nd gen
1st gen = simple, flexible molecule
2nd gen = more complex, bulkier with more func groups
2nd gen H1 antihistamiens
tefernadine (seldane) and astemizole (hismanal)
non-sedating
removed from market in 1997 (terfenadine) and 1999 (astemizole) b/c could potentially cause fatal heartbeat irregularities when taken with certain drugs and foods
ketoconozole (extina, kuric) and erythromycin (erythrocin)
interfered with drug metabolism by inhibiting CYP3A4
increased concentration of terfenadine or astemizole in bloodstream
fexofenadine (allegra)
acrivastine
used for allergic rhinitis in combo with pseudoephedrine (benadryl)
loratidine (claritin)
desloratadine (clarinex) → rx only
cetirizine (zyrtec)
levocetirizine (r-cetirizine)
nasal sprays or opth solutions
azelastine hydrochloride
nasal sprays available OTC (astelin)
ophthalmic (optivar)
olopatadine hydrochloride
ophthalamic, available OTC (patanase, patanol)
epinastine (elestat) → ophthalmic
use and level of efficacy of antihistamines
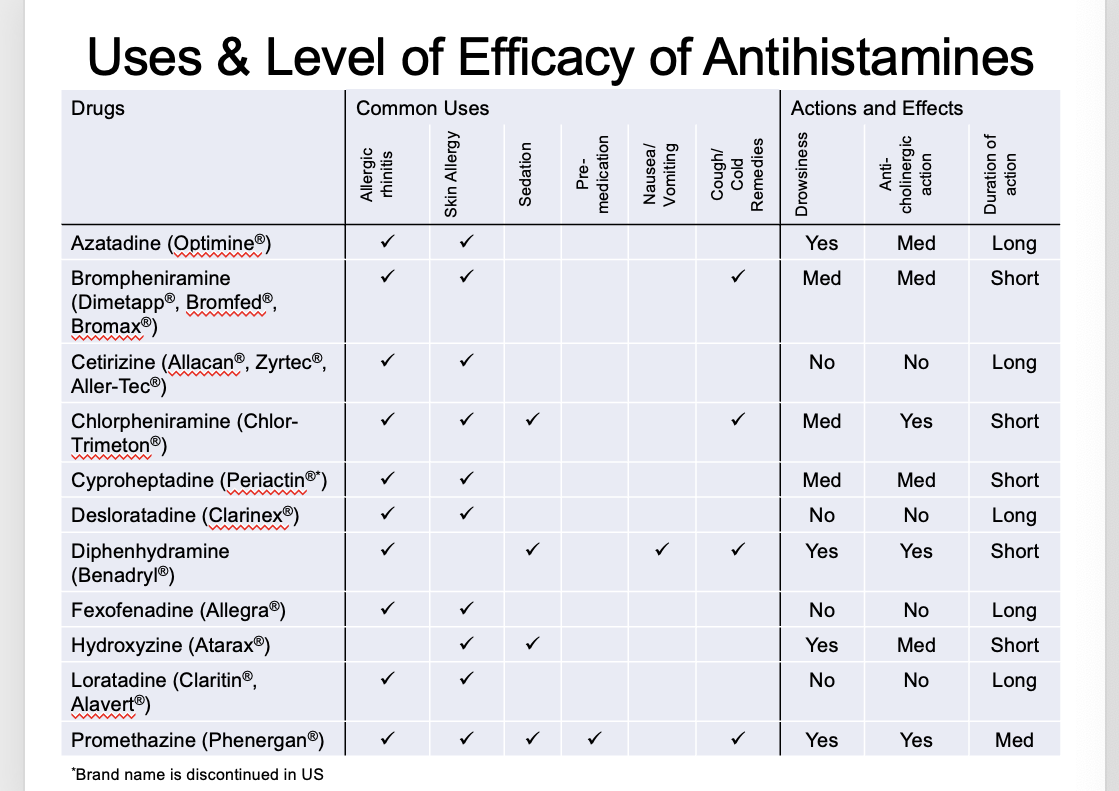
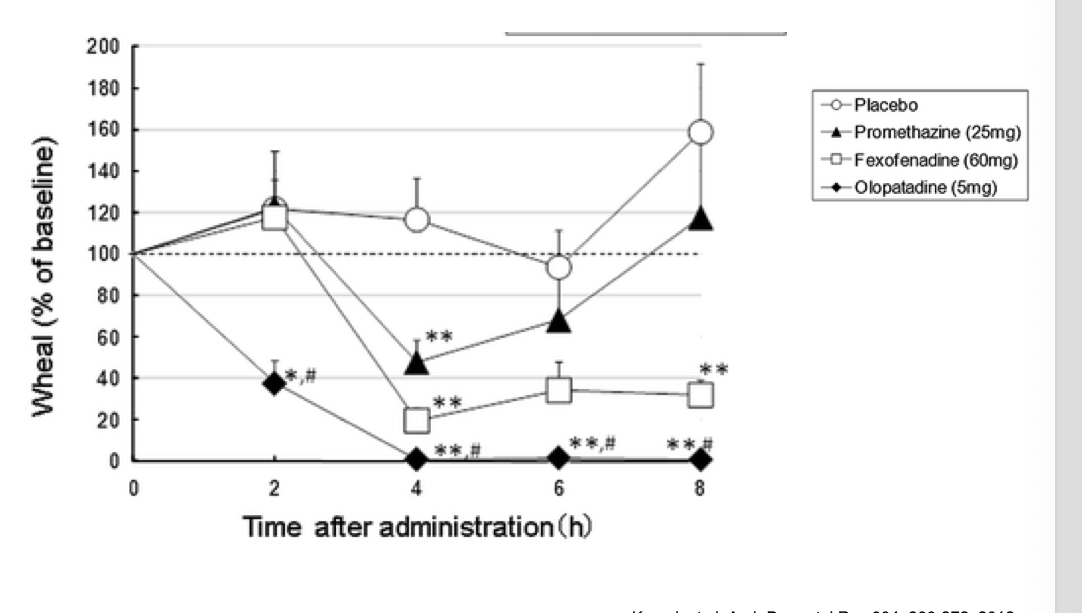
level of efficacy of antihistamines: skin responses
wheal = skin swelling
placebo → wheal size remainsnear baseline throughout
promethazine (1st gen) → some reduction but then starts to go back up
fexofenadine (2nd gen)→ reduction and stays down
olopatadine (2nd gen) → even greater reduction (basically none) and stays down
toxic rxns and side effects of H1 inverse agonists
CNS depression (mainly found in 1st gen)
allergic reactions (topical application)
appetite loss, nausea and vomiting, constipation or diarrhea
insomnia, tremors, nervousness, irritability
CNS stimulation with hallucinations, motor disturbances (tremors and convulsions) and death
secreted in breast milk → can cross placenta
drug interactions of H1 inverse agonists (predominantly 1st gen)
H1 antihistamines that produce sedation can potentiate the effects of CNS depressants
e.g. barbituates, opiates, general anesthetics, alcohol
H1 antihistamines that possess anticholinergic actions can produce manifestations of excessive blockade if given with anticholinergic drugs
e.g. dry mouth, constipation, blurred vision
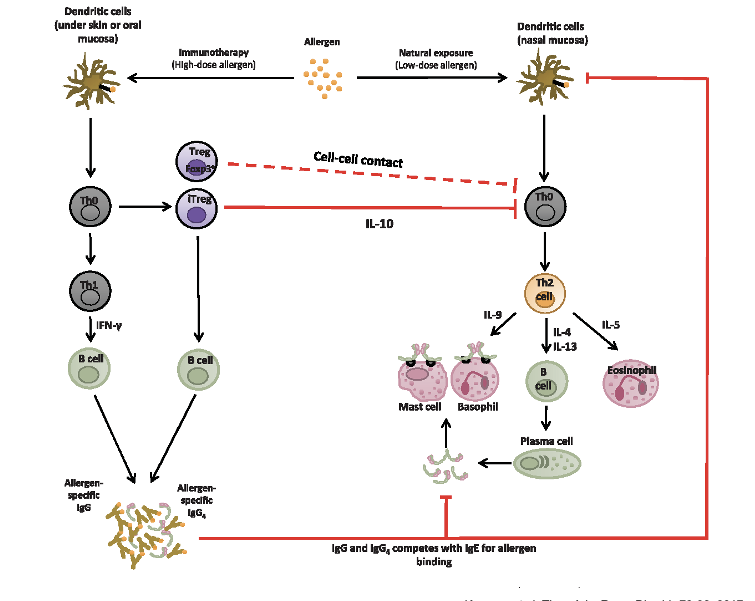
other allergy treatments - sublingual/subq desensitization
2 routes of allergen exposure
high-dose immunotherapy
allergen goes to dendritic cell (under skin or oral mucosal) → DCs process and present allergen to Th0
Th0 → Treg → IL-10 production that suppresses allergic response; direct Treg-DC interaction enhance tolerance induction (bidirectional feedback loop)
Th0 → Th1 → B cell → allergen specific IgG → IgG competes with IgE for allergen binding (competition prevents IGE from triggering mast cell and basophil degranulation aka no histamine released)
other allergy treatments
omalizumab (xolair)
anti-iGE
originally approved in 2003 for aasthma
now approved for protection against accidental exposure to food allergens in patients 1 yr and older
h2 antagonist mechanisms and pharmacologicla effects
displaces histamine from H2 receptor
H2 blockers lead to decrease in cAMP and Ca2+
competitive, reversible antagonists of the H2 receptors
inhibit secretory function of gastric mucosa parietal cells induced by histamine, gastrin, and pentagastrin
reduces gastric acid volume + concentration of pepsin
few other effects
h2 receptor antagonists
available OTC
famotidine (pepcid)
cimetidine (tagamet) → HAS MOST SIDE EFFECTS
ranitidine (zantac) → removed from market 4/2020 due to presence of N-nitrosodimethylamine
nizatidine (axis)
in most cases, replaced by H+ pump inhibitors
adverse effects
diarrhea
dizziness
headache
somnolence
rash
constipation
vomiting
arthralgia
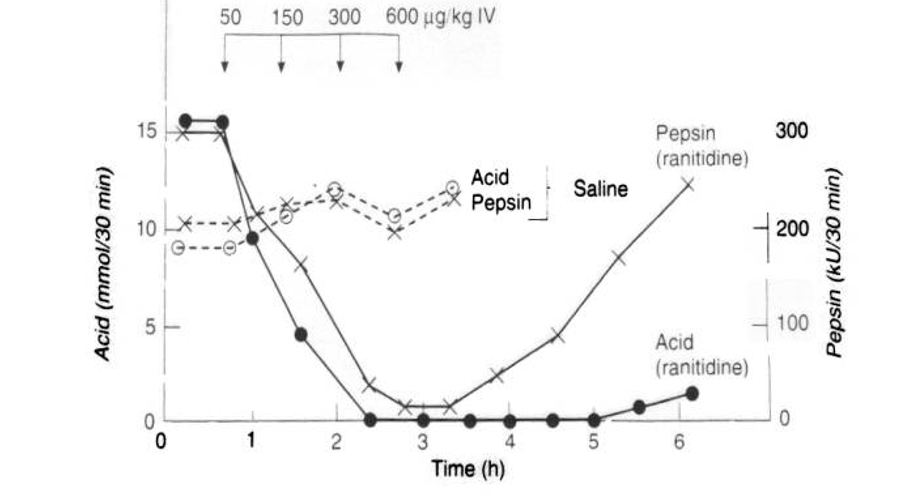
effects of H2 receptor antagonists on gastric acid secretion
ranitidine
dramatic suppresion of acid secretion
pepsin secretion decreases but then goes back up as time goes on goes on
h2 antagonist therapeutic uses
duodenal ulcer
gastric ulcer
zollinger-ellison syndrome = a pathological hypersecretory state resulting in excessive gastric pepsin and HCl, usually fatal, gastrin secreting tumor
gastroesopheageal reflux disease
used prior to surgery in pts with GI obstruction to elevate gastric pH
reflux esophagitis
antacid
effects NOT due to H2 receptor blockade
off-target pharmacological effects
inhibition of cytochrome P-450 oxidative drug metabolizing system (cimetidine, ranitidine)
inhibition of 1st pass gastric metabolism of ethanol (cimetidine, ranitidine, nizatidine)
inhibition of acetaminophen glucoronidation (ranitidine)
inhibition of renal clearance of basic drugs that are secreted by the renal tubule (cimetidine, ranitidine)
anti-androgen effects (cimetidine)
toxic rxns of H2 antagonists (mostly associated with cimetidine)
most common (1-2% of pts)
diarrhea
dizziness
somnolence
headache
rash
CNS effects
slurred speech
delirium
confusion
most commonly seed in older pts or thoes with liver or kidney impairment
endocrine function (minor and reversible)
anti-androgen effects (e.g. loss of libido, impotence, reduced sperm count)
blood dyscrasias (abnormalities in blood cells)
liver
reversible cholestasis
reduced blood flow
agents that inhibit gastric secretion alter the bioavailability and rate of absorption of many other drugs
H3 receptor drugs
believed to act as feedback inhibitors in a wide variety of organ systems and in the CNS
agonists cause sedation
antagonists improve cognition
GI
agonists down-regulate histamine
decrease gastrin
lung
agonists have a bronchodilatory effect
allergic rhinitis
antagonists improve cognition and memory, act as antipsychotics
agonists display anti-nociceptic activity (reduction of painful stimulus)
potential clinical uses
narcolepsy
antipsychotics
anti-allergy drugs (in combo with H1/H3)
anti-obesity drugs (preclinical, ciproxifan analog)
examples of H3 receptor drugs
betahistine (serc)
used in treatment of meniere’s disease; severe motor intolerance; treatment of vertigo associated with vestibular (includes inner ear) loss (H1 agonist; H3 antagonist)
approved in europe
NOT FDA approved
can be obtained with rx from compounding pharmacies
pitolisant (wakix)
inverse agonist
used to treat narcolepsy
FDA approved
H4 receptor antagonists
1-[(5-chloro-1H-indol-2-yl)carbonyl]-4-methylpiperazine
(JNJ 7777120)
experimental antagonist
involved in immune and inflamatory responses rather than gastric acid secretion or neurotransmission
![<ul><li><p><span>1-[(5-chloro-1H-indol-2-yl)carbonyl]-4-methylpiperazine</span></p><p><span>(JNJ 7777120)</span></p></li><li><p><span>experimental antagonist</span></p></li><li><p><span>involved in immune and inflamatory responses rather than gastric acid secretion or neurotransmission</span></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/aa5e6050-3c0c-452d-898a-c58259c2320a.png)
effects of H4 antagonists
block histamine-induced chemotaxis (directed movement of immune cells towards a rising concentration of histamine) and Ca2+ influx in mast cells
block the histamine-induced migration of tracheal mast cells from the connective tissue → epithelium
block neutrophil infiltration in a mouse zymosan-induced peritonitis model
ameliorate allergen induced, Th2 cytokine driven pathologies in a mouse model of allergic asthma, including lung remodeling and airway dysfunction
reduce pruiritus induced by histamine, substance P, or 2,4-dinitrochlorobenzene in mouse models
antagonist, N-(2-aminoethyl)-5-chloro1H-indol-2-carboxnamide, is effective against allergic asthma in mice
antagonist, JNJ7777120, is effective against diabetic retinopathy in mice
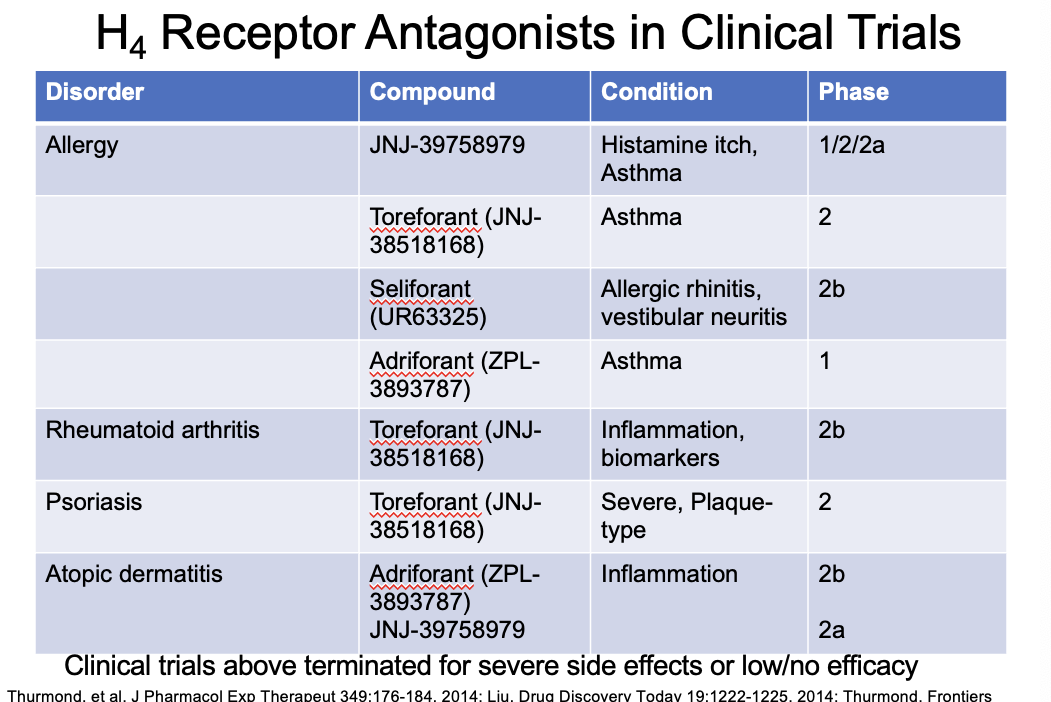
H4 receptor antagonists in clinical trials
terminated for severe side effects or low/no efficacy
H4 agonists
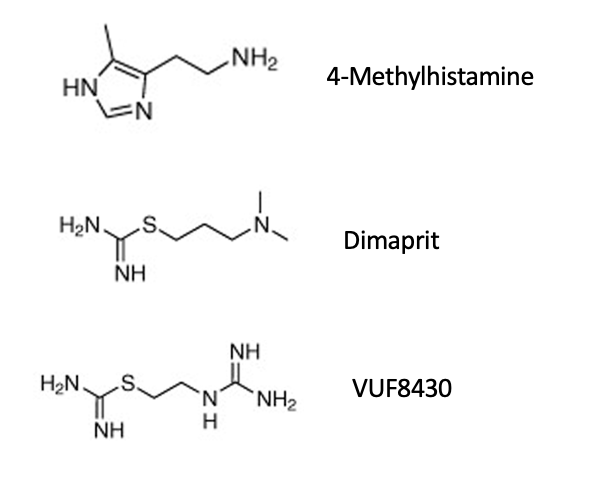
4-methylhistamine
dimaprit
VUF8430
agonists = pro-inflammatory; can induce pruritis in mice