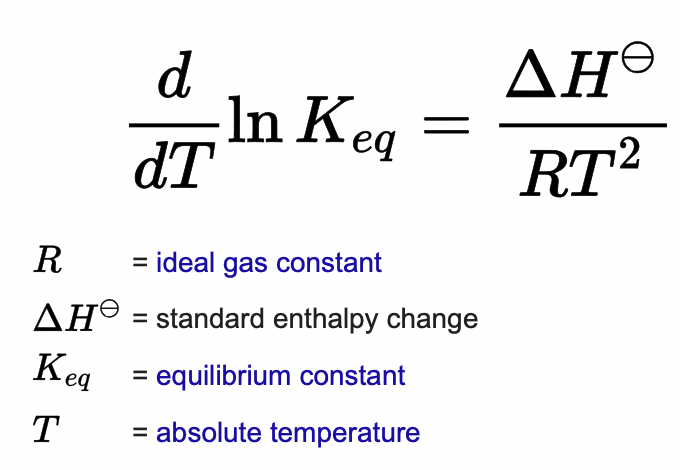7&8 - physicochemical properties of drugs
1/19
Earn XP
Description and Tags
Dr Alison lansley
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
What are physicochemical properties
Physical and chemical properties of drugs eg molecular formula, weight, physical state, appearance, colour, odour, evaporation rate, density, particle size, solubility, acid dissociation constant, boiling and melting point, vapour pressure, partition coefficient
Why are physicochemical properties important
Info needed so drugs can be formulated into dosage forms. The physicochemical properties properties of the API will determine whether a particular dosage form or route of administration are viable
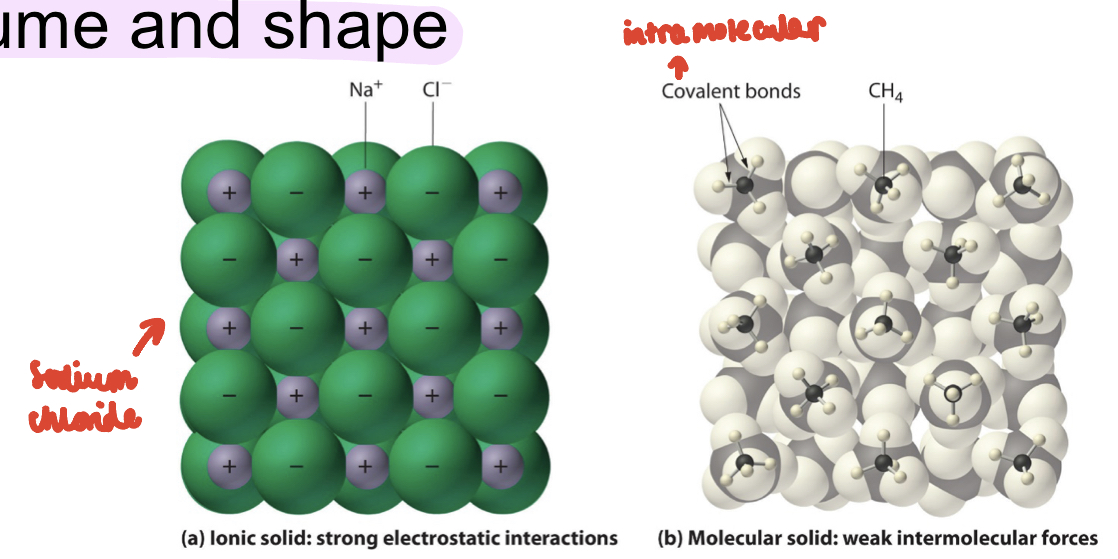
How are solid molecules held
-held in fixed positions by intermolecular forces and have a definite volume and shape
-intermolecular forces include: hydrogen bonds, VdW forces
What’s the structure of crystalline solids
-arranged in a define order which is repeated throughout the crystal
-sharp well defined melting points
-most drugs form triclinic, monoclinic or orthorhombic unit cells
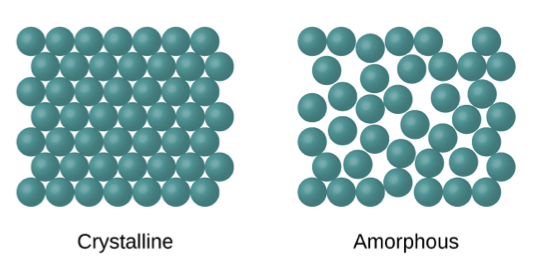
How are amorphous solids arranged
-does not have the long range order of a crystal
-the molecules are in a random arrangement
-amorphous form is usually more soluble than crystalline form
What is polymorphism and why is it important
-Crystalline forms of the same drug. Chemically the same but with different structures at a molecular level/different packaging patterns eg carbon has polymorphs of diamond graphite
-types of polymorphs: ‘enantiotropic polymorphs’ can convert from one form to another under changing conditions eg. Temp and pressure. ‘Monotropic’ one form stable at all conditions. ‘Metastable’ will convert to preferred form eventually and is less stable, often preferred bc more soluble and dissolves faster, improved bioavailability.
-important as we need to identify, understand and be able to quantify this inherent instability in drugs because: different polymorphs can have different physical properties so can behave differently when being processed/made and also in the body
what are crystal hydrates
crystals that contain water molecules in crystal lattice in an exact molar ratio: 1mol drug and 1mol water - monohydrate. 1mol drug and 2mols water - dehydrate.
crystal hydrates described as pseudopolymorphs bc they have different properties to the anhydrous form
whats the structure of a crystal hydrate
-lattice held more tightly in hydrate
-requires more energy to dissolve
-hydrate often has: higher MP, lower solubility, lower dissolution rate THAN the equivalent anhydrous form
what is the crystal lattice energy
defined as the energy released when the constituent atoms are placed in their respective positions on the crystal lattice
can also be defined as energy used to separate ionic crystal into its constituent ions
relates to strength of interactions between ions and molecules
relates to melting point and dissolution
what is the definition of a solution
mixture of two or more components that form a single phase which is homogeneous down to the molecular level
solute dissolved in a solvent (water) forms a solution
dissolution definition
transfer of molecules or ions from solid state into a solution
solubility definition
the extent to which dissolution proceeds. amount of dissolved solute in equilibrium with undissolved solid
when is a solution saturated
when a solution contains the solute at the limit of its solubility then the solution id saturated
what is the solubility of drug molecules affected by
-lipophilicity (attraction to fats) of molecule (logP)
-size: molecular weight and shape of molecule
-pKa of molecule
-crystal lattice energy of molecule
what are the factors affecting dissolution?(dissolution is the process where a solute in gaseous, liquid or solid phase dissolves in a solvent to form a solution
-wetting
-wettability of a drug estimated based on its contact angle with water
what is contact angle?
contact angle is the angle between a liquid/vapour interface and a substrate surface
hydrophobic materials- contact angle >90' with water
hydrophilic materials- contact angle <90 with water
if contact angle close to zero - slightly wetted
what is a hygroscopic material
something that pulls water into itself, must be protected from the environment by packaging
what is the notes-whitney equation
The Noyes Whitney equation, dC/dT = kS(Cs-Ct) describes the change in concentration over time (dC/dT) as a function of the dissolution rate constant k, surface area S, solubility Cs, and concentration in the bulk fluid Ct.
what is the van’t Hoff equation
The van 't Hoff equation relates the change in the equilibrium constant, Keq, of a chemical reaction to the change in temperature, T, given the standard enthalpy change, ΔH⊖, for the process.
the equation predicts:
-solubility increases with temperature(ideal solutions)
-solubility decreases as melting point increases(ideal solutions)