Chemistry Quiz 1
1/82
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
83 Terms
Significant figure Rule 1
Non-zero integers are ALWAYS significant
Significant figure Rule 2
Leading Zeros are NOT significant (zeros to the left)
Significant figure Rule 3
Trailing Zeros are significant only if there is a decimal point present.
Significant figure Rule 4
Captive zeros are always significant
Rounding Numbers Rule 1
#>5 round up
Rounding Numbers Rule 2
#<5 round down
Rounding Numbers Rule 3
#=5 if the number being rounded is even keep it the same, if it is odd round up
Multiplication and Division Rule
Answer contains the same amount of Significant figures as the number with the least significant figures.
Addition and Subtraction Rule
Answer has the same # of decimal places as the number with the least decimal places.
Density
Ratio of the mass of an Object/substance to the volume of the object/substance
Density formula
d=mass/volume
Dimensional Analysis
Starting quantity x Conversion factors = final quantity
Prefix for SI units we use
Mega, Kilo, Deci, Centi, Mili, Micro, Nano
Mega
M 106
Kilo
k 10³
Deci
d 10-1
Centi
c 10-2
Mili
m 10-3
Nano
n 10-9
Micro
µ 10-6
Nuclide Symbol
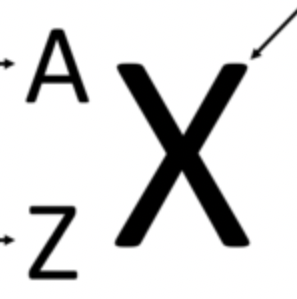
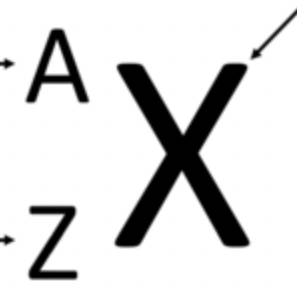
What is A
Mass Number (A=P++No)
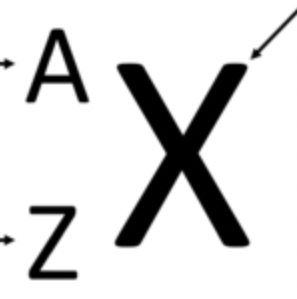
What is z
Atomic Number (P+=e-)
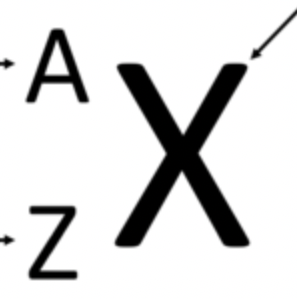
What is X
Atomic symbol
How to fine No
No=A-Z
Metals
Generally shiny solids at room temp (Except mercury) Conduct heat and electricity well
Nonmetals
Generally gases or dull, brittle solids at room temp, does not conduct heat or electricity well.
Metalloids
Between metals and nonmetals
Group 1
Alkali Metals (Highly Reactive)
Group 2
Alkaline Earth Metals (Highly Reactive)
Group 17
Halogens (Nonmetal but reactive)
Group 18
Noble gases (Stable nonmetals)
Group 13-16
Named after first element in group
Covalent compound
Electrons are shared between atoms of different elements
Ionic compound
Electrons are transferred from one element to another
Molecular compound
Elements held together by a covalent bond (Non metals)
mono
1
di
2
tri
3
tetra
4
penta
5
hexa
6
hepta
7
octa
8
nona
9
deci
10
Group 1 charged Ions
#+
Group 2 charged Ions
#2+
Group 3 charged Ions
#3+
Group 11 charged Ions
#+
Group 12 Charged Ions
#2+
Group 13 charged Ions
#3+
Group 15 charged Ions
#3-
Group 16 Charged Ions
#2-
Group 17 Charged Ions
#-
Cr2+
Chromium (II) or Chromous
Cr3+
Chromium (III) or Chromic
Co2+
Cobalt (II) or Cobaltous
Co3+
Cobalt (III) or Cobaltic
Cu+
Copper (I) or Cuprous
Cu2+
Copper (II) or Cupric
Fe2+
Iron (II) or Ferrous
Fe3+
Iron (III) or Ferric
Pb2+
Lead (II)
Pb4+
Lead (IV)
Hg22+
Mercury (I) or mercurous
Hg2+
Mercury (II) or Mercuric
Sn2+
Tin (II) or Stannous
Sn4+
Tin (IV) or Stannic
NH4+
Ammonium
NO2-
Nitrite
SO32-
Sulfite
H3O+
hydronium
NO3-
Nitrate
SO42-
Sulfate
C2H3O2-
acetate
CO32-
Carbonate
CN-
cyanide
HCO3-
hydrogen carbonate
OH-
hydroxide
PO43-
phosphate
Avogadros Number
6.022 × 1023
Solution
(aq)