Oral Solid Dosage Forms
1/100
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
101 Terms
What is a tablet?
A solid dosage form
It comprises the API and excipients, all compressed into a single unit
All components are solids
What is a capsule
A solid dosage form
It comprises an API and excipients loaded into a shell, which may be hard or soft
The contents of the capsule may be solid or liquid
2 different types of capsules
Hard-shell capsules:
Comprise two pieces, a body and a cap
Made of e.g. starch or hydroxypropylmethylcelluose
May have solid or liquid contents
Soft shell capsules:
Consist of a single-piece shell, typically made of gelatin
The contents are liquid
Why do we use solid dosage forms?
Convenient and accurate dose
Increased drug stability
Simple and easy administration
Altered drug release rates - IR or MR
Mass production
Advantage of solid dosage forms in the context of doses
Can make a wide range of doses and a wide range of drug release profiles
2 problems with making tablets and capsules
Powder formulation
Segregation
Problems with tablets and capsules
Powder Flow
Most APIs are crystalline materials
This means the particles are irregular shaped
This means they usually have poor flow properties
So there is a lot of powder flow when making both tablets and capsules
Problems with tablets and capsules
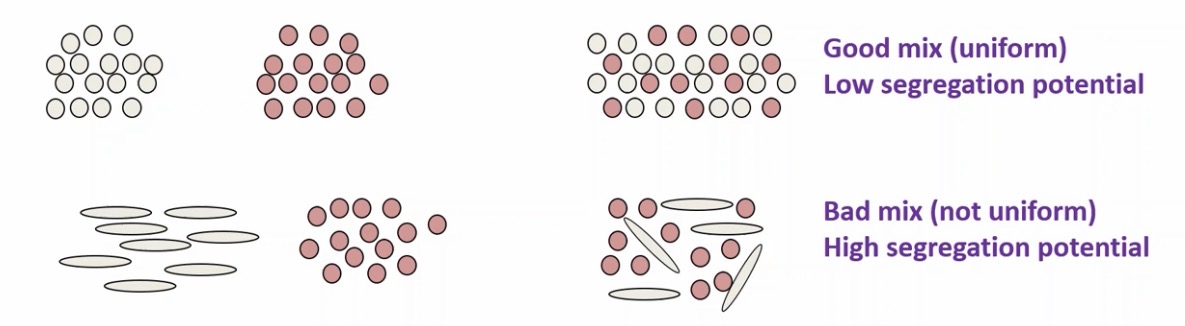
Segregation
Have to prevent segregation of different powders ie. Excipients and APIs
To avoid this, particles must be the same size and shape
Problems with tablets and capsules
How can we solve the problems of powder flow and segregation?
Granulation
Particles of varying shape and size are gathered into larger, uniform and permanent aggregates
In which the original particles can still be identified

Problems with tablets and capsules
Granulation
We seek to make granules which…
Have a narrow particle size distribution and a shape close to spherical
Are easily fluidised and flow well
Are easily compressible, and stable when compressed » for making tablets
Are produced by a robust and reproducible process, with a clear end-point
Problems with tablets and capsules
Granulation
Benefits of granulation
To improve flow properties of the material.
To prevent segregation of the constituents in a powder mix (content uniformity).
To increase bulk density = improves flow
To reduce dust production (hazard)
To improve compression characteristics
To improve* dissolution rate » faster OR slower depending on desired properties
For control of moisture content
Problems with tablets and capsules
Granulation
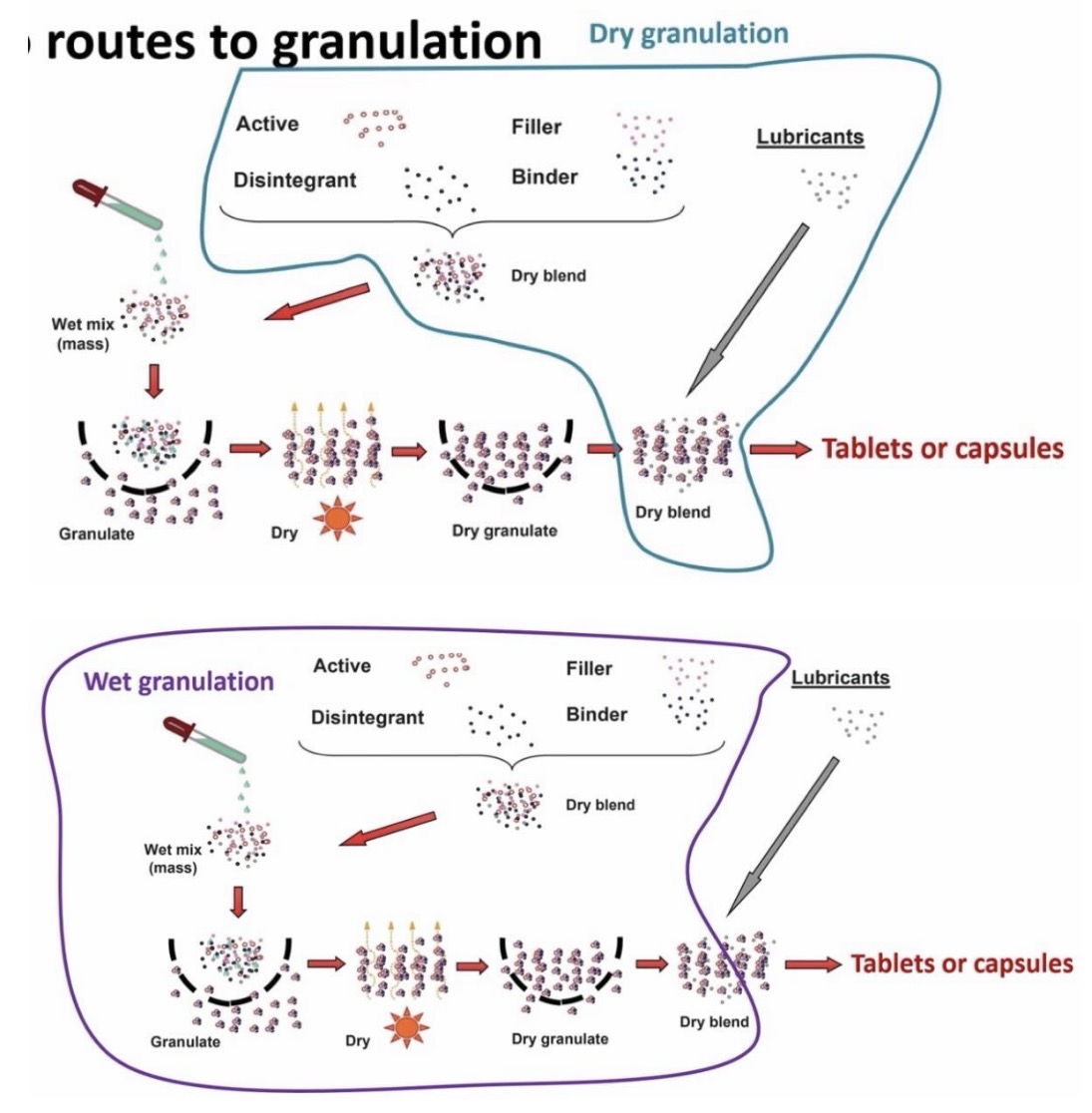
Components
2 routes of granulation
Powders:
API
Filler - provides bulk to the tablet
Binder - sticks together API and excipients
Disintegrant - breaks tablet/capsule up in the body, releasing drug
Dry granulation
Wet granulation
Problems with tablets and capsules
Granulation
Wet vs Dry granulation
Wet:
Commonly used as more likely to produce good granules
If needed, we can do wet granulation in solvents other than water, but water is preferred. We use the minimum amount we can
Dry:
Dry granulation is necessary when the ingredients are sensitive to moisture or heat
Problems with tablets and capsules
Granulation - Wet
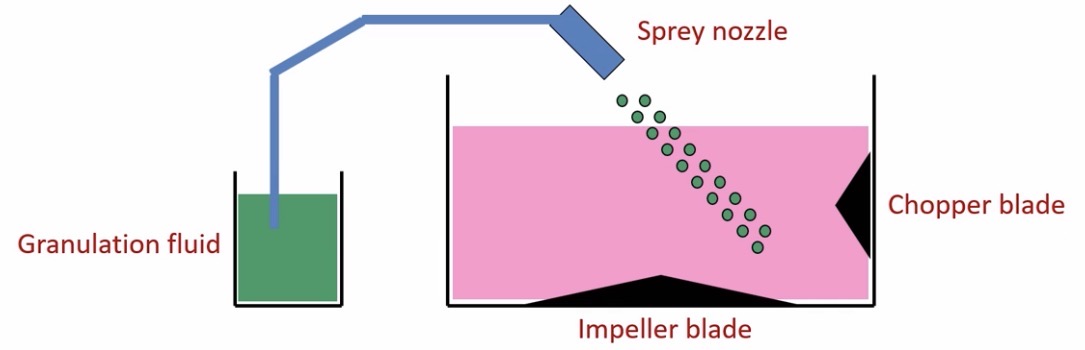
Process
Start with materials that have been ground to the same size
A binder is then added:
Either added to the drug and diluent as a powder mix followed by mixing with water (dry binder)
or as a pre-formed solution (wet binder)
Binders are typically polymers like sucrose, starch, celluloses etc.
Granulating fluid added to make the wet mass
Chopper blade turns to break big lumps up
Problems with tablets and capsules
Granulation - Wet
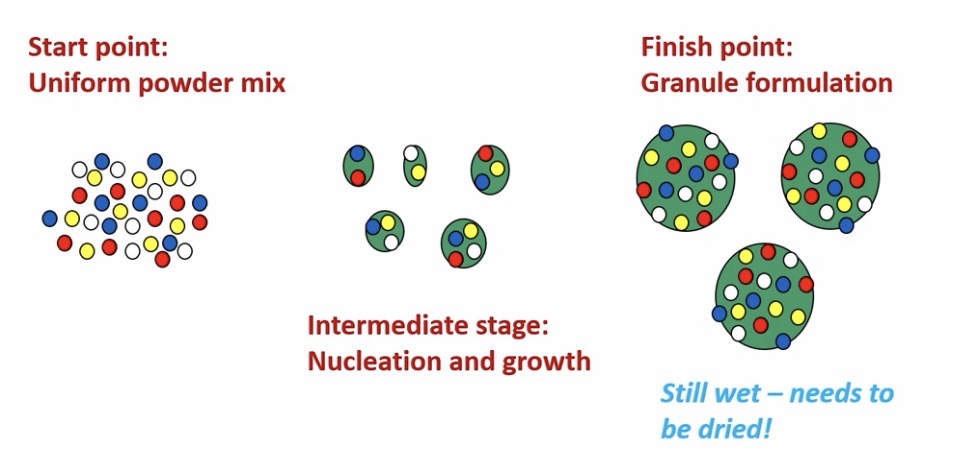
What does the granulation fluid do?
Sticks particles together in small aggregates
Which then forms bigger granules
Once granules are big enough, they are dried
Problems with tablets and capsules
Granulation - Wet
Properties of dried granules
Porous structure
Solid bridges between particles in the granules
Problems with tablets and capsules
Granulation - Wet
What does it mean if dried granules are more porous?
More porous granules = dissolve faster BUT weaker mechanical strength
Problems with tablets and capsules
Granulation - Wet
How can we control the porosity and bridges between particles in the dried granules?
By controlling:
Amount of binder added
The length of time particles are granulated for
The concentration of the powders in the system
Problems with tablets and capsules
Granulation - Wet
When can wet granulation go bad?
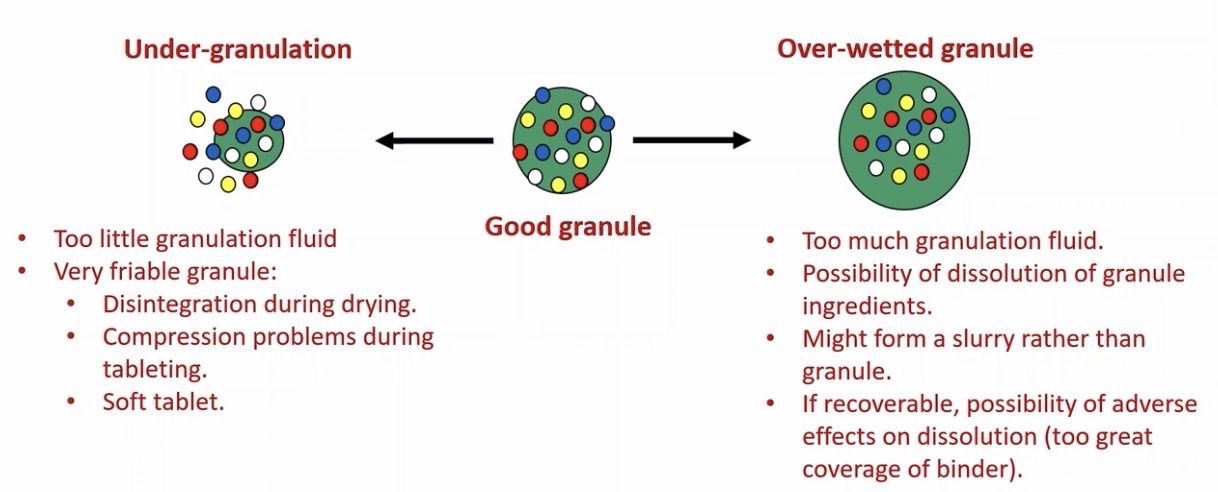
Problems with tablets and capsules
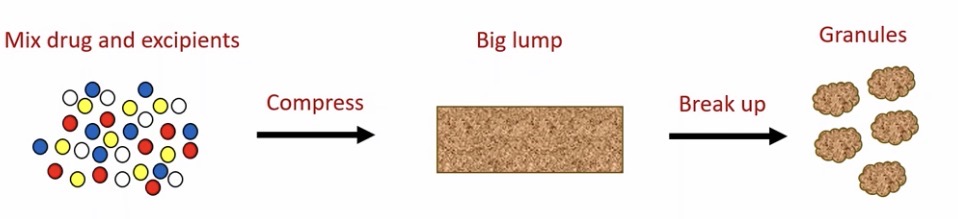
Granulation - Dry
Process
Mix drug and excipients
Lubricant is added » helps powder flow through tablet machinery effectively
Compressed to form a big lump
Lump is broken up into granules
Problems with tablets and capsules
Granulation - Dry
Why is this process not very good?
Granules produced are not very regular and much less spherical = poor flow properties
Problems with tablets and capsules
Granulation
Pros and cons of wet granulation
Pros:
Increases granule strength
Can be used with a wide range of API concentrations
Useful for APIs with poor flow properties
Uniform distribution of drug and different colours
Prevents segregation
Produces tablet that are hard, non-friable and easy to coat
Cons:
Many stages
Long process times
Expensive equipment
Potentially hazardous dust
Requires solvents » bad for water soluble drugs
Drug is heated » bad for drugs which are not heat stable
Migration of soluble components to granule surface
Problems with tablets and capsules
Granulation
Pros and cons of dry granulation
Pros:
Less equipment so cheaper
No expensive drying process
No binders
No heat or liquid » good for sensitive APIs
Cons:
Multiple steps
Produces irregular granules so poor powder flow
Poor colour distribution
Hazardous dust production
Final tablets are softer than those from wet granulation and harder to coat
2 routes of making a tablet
Powder —> Granules —> Tablets (via granulation, then compression)
Powder —> Tablets (via direct compression)
Making Tablets
How do you make a tablet?
Take a powder and squash it:
Die is filled with powder
Lower punch is moved up and down to control how much powder fills the die
Pressure is applied from upper punch and lower punch to squash all powders together to form a single unit
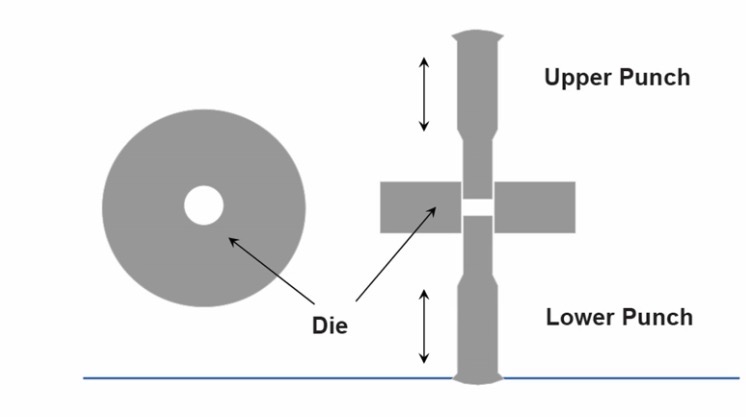
Making Tablets
What machine is used to make tablets in large-scale manufacturing?
Multi-station press
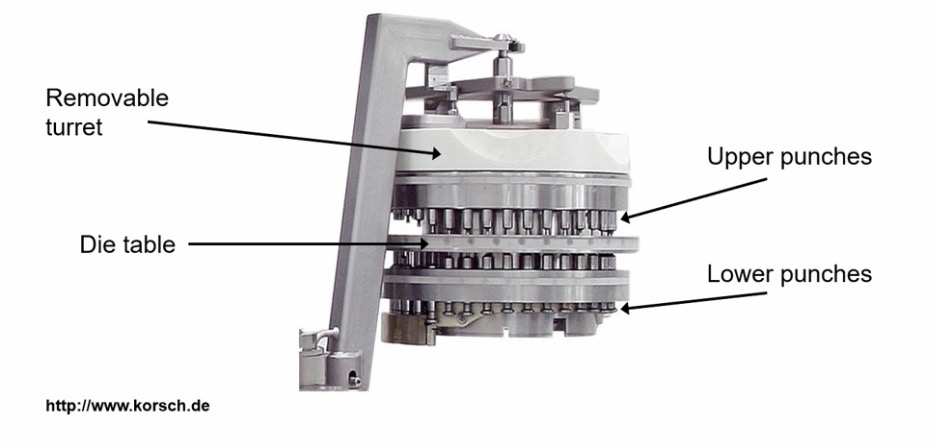
Making Tablets
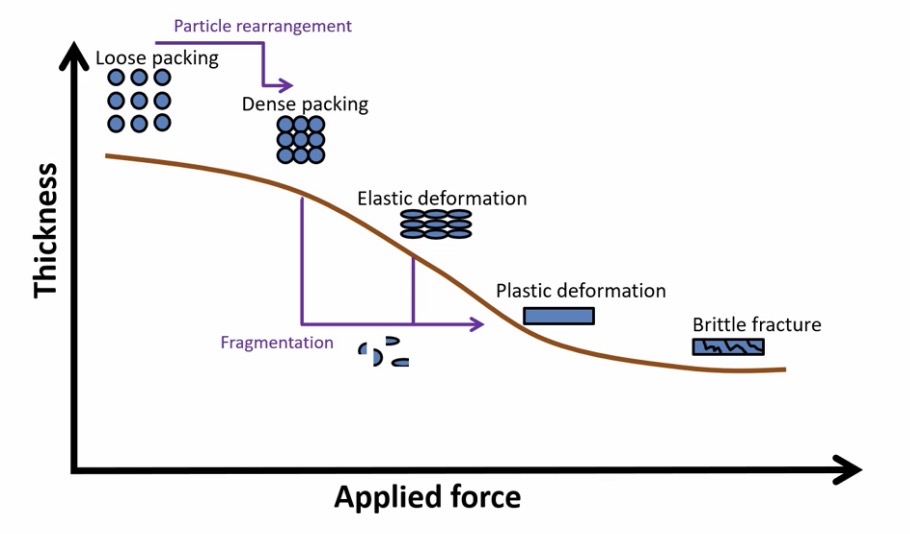
Stages of powder compaction
As pressure is applied:
Loose packing
Dense packing - air removed, particles pushed close together
Elastic deformation - shape of particles changes, reverses when pressure is removed
Plastic deformation - permanent change in shape, tablet is formed
However, if too much pressure is applied:
Brittle fracture - tablet breaks into pieces
By which forces are tablets held together?
Non-covalent adhesive forces between particles - Van der Waals forces
2 types of compression characteristics of the drug/granules
Brittle fracture on compression
Particles break on compression
This creates a larger surface area for adhesion
Plastic deformation on compression
Shape of particles changes permanently on compression
Does not increase surface area
» we need to have a balance of these in our tablet
Why do we need both brittle fracture and plastic deformation in our tablet?
Brittle fracture leads to more inter-particulate bonding = produces stronger tablets
BUT the tablets are harder to remove from the die, and there may be problems with distintegration and drug release in the body
Plastic deformation gives less inter-particulate bonding = produces weaker tablets
BUT the tablets are easier to remove from the die, and have fewer dissolution problems
We want a balance between brittle fracture and plastic deformation to get optimum tablets
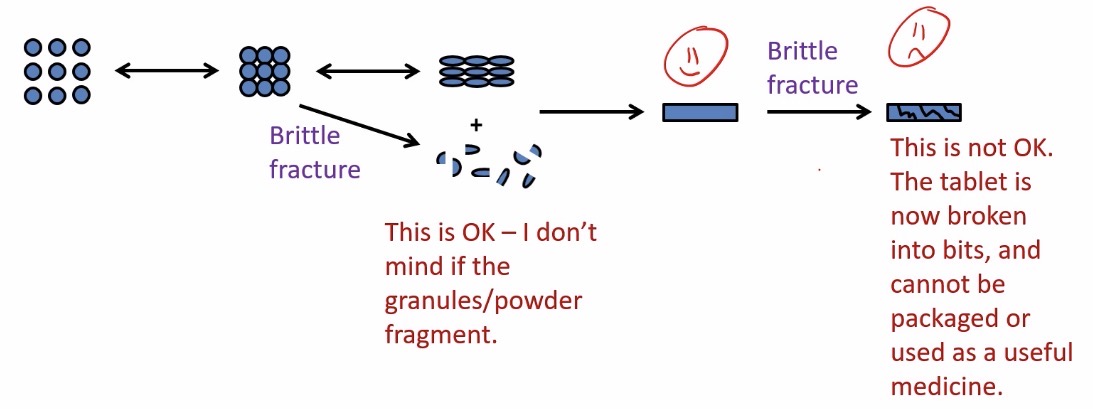
Both the tablets and granules/powder can undergo brittle fracture. Explain what this means
When pressure is applied, before a tablet is formed, some of the powder/granules might fragment » this is completely okay for the tablet formulation
However, once the tablet is formed, if more pressure is applied the tablet will break up » not okay as cannot be packaged or used
Criteria of the powder and tablets for successful tablet formulation
The powder must:
Flow well - to give a consistent tablet weight.
Not segregate - to give a consistent dosage per tablet
Be compressible - to form robust tablets.
Be lubricated - to avoid sticking in the tableting machine.
And the tablets must:
Disintegrate - i.e. break up to release the drug.
Release the drug - determined by dissolution testing.
Be fit for purpose - tablets that the patient can handle!
What powder criteria does granulation allow us to achieve and why (summary)
Allows the powder to:
Flow well » forms spherical particles
Not segregate » api and excipients stuck together
Be compressible » right balance of brittle fracture and plastic deformation
How can tablets be made without granulation?
Direct compression
Making Tablets
Direct Compression
Direct compression is used if the drug is of low dosage and will flow well
We add a compression aid - a bulking agent with good flow and compression properties
Examples of compression aids include:
Microcrystalline cellulose (Avicel®) = plastic deformation excipient
Dicalcium phosphate (Emcompress®) = brittle fracture excipient
Spray dried lactose = brittle fracture excipient
Making Tablets
Direct Compression
Pros and Cons
Advantages:
Two step process » blend and compress
Improved stability
No heat
No water
Disadvantages:
Require specialist (spray-dried) excipients = expansive
Poor flow/compression properties
Use of flow aid.
Segregation (uniformity of dosage form)
Size and density of API and excipients may be different = segregation
What is a film coat?
Thin outer layer that covers a tablet core
Film coatings are usually either:
Sugar
Polymer
Why do we film coat?
As a physical barrier:
To protect the drug (moisture, light, O2 etc)
To protect the user (some drugs are very potent)
For identification:
Patients/HCPs can quickly identify a tablet based on colour
Taste-masking
To improve handling - if tablet is friable (easily broken)
To modify the rate of drug release
Example of very potent drugs that are film coated to prevent patient overdosing on these drugs e..g by licking fingers
Finasteride
Chlorambucil
What is an enteric film coat?
Where a film coat is designed to delay or modify drug release (usually by a polymer)
If we did not want a certain drug to dissolve in the stomach acid, what would we make the film coating out of?
A polymer with pH-dependent solubility
If a tablet is ‘gastro-resistant’…
This means it has an enteric coating
What should you tell patients when giving tablets which are enteric-coated?
Do not break tablet in half
Do not crush tablet
Do not put tablet in juice etc (acidic = causes drug to be released early)
How can you tell whether a film coating of a tablet is made of sugar or polymers?
Sugar film coating are thicker than polymers
Sugar Film Coating
Properties
E.g. like M&Ms
Thick coat (0.5-2mm) that covers imperfections
Gives a shiny finish
Masks bitter taste
Not used to control drug release » sugar dissolves quickly at any pH
Sugar Film Coating
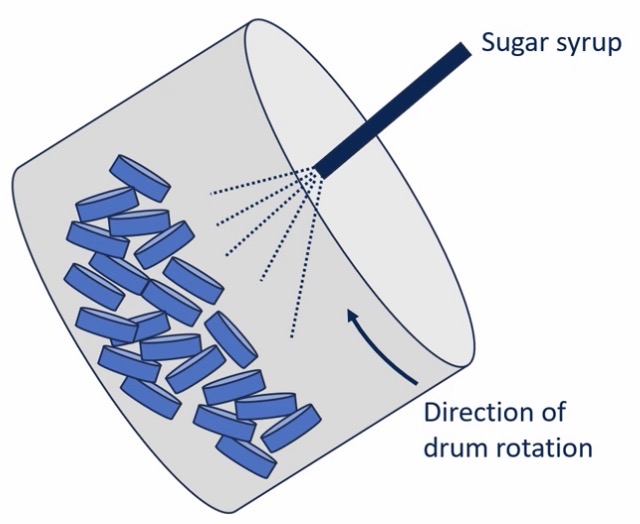
How is it made?
Tablet cores are placed in a rotating drum
Sugar syrup is sprayed into the drum
Usually multiple layers of sugar coating:
Seal
Sub-coat(s)
Smoothing coats)
Colour coat
Polishing coat
Printing coat (optional)
Polymer Film Coating
Properties
Thin coat (20-200 um) that does not cover imperfections
Gives a matte finish
Masks bitter taster
Can control drug release » polymer has pH dependent solubility
Polymer Film Coating
Basic film coat
No pH-dependent dissolution
Typically comprises:
HPMC » forms a high strength film
HPC » gives good flexibility
Titanium dioxide » makes the film opaque
Dye
» all dissolved in water
Polymer Film Coating
Enteric film coat
Designed to be insoluble at low pH
Typically comprises:
Polymer - Cellulose acetate phthalate (Aquateric) OR Polymethacrylates (Eudragit)
Plasticiser (like triacetin or PEG) » goes between polymer chains and makes the film more flexible
» Polymers are insoluble in water at certain pHs, so dissolved in ethanol
Polymer Film Coating
How is it made?
Tablet cores are placed in a porous/perforated rotating drum
Hot air enters to evaporate the ethanol
Holes in the drum to let ethanol evaporate
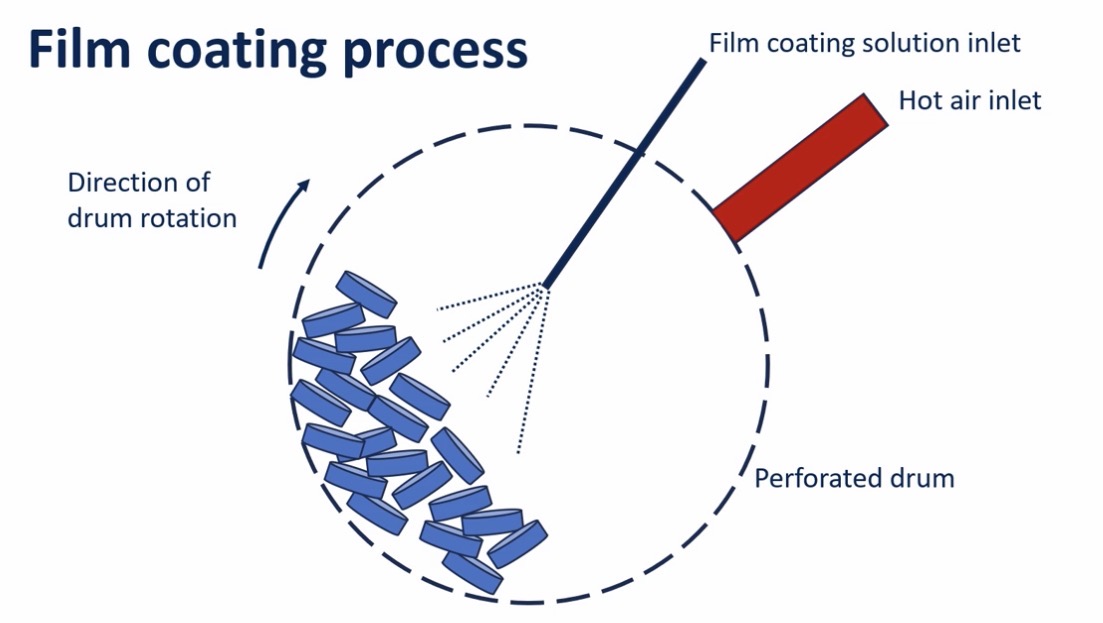
Why is a porous drum used in polymer coating but not sugar coating?
In polymer coating, solvent used is ethanol
Pores allow ethanol to evaporate
What is drying?
The removal of water (or another solvent) to form a solid
What samples can be dried?
Wet solid
Suspension
Solution
Are materials ever completely dry?
No
Water may still be on the surface or within cells
Water is important for materials for maintaining their structure
Why do we dry materials?
To improve physical properties e.g. powder flow, compressibility » dry powders will flow better than wet powders (less clumping)
To improve stability by reducing:
Hydrolysis
Polymorph conversion
Growth of microorganisms
Reducing efflorescence » fat crystallising out of a material
Why do we not completely dry materials?
Some water content is needed for good compression
4 methods of drying
Tray-drying
Fluidised-bed drying
Spray-drying
Freeze-drying
Tray Drying
Method
Advantages
Disadvantages
Sample (wet powder) is spread thinly on a tray in a convective oven
Hot, turbulent air is then passed over the sample
Advantages:
Cheap and easy to use
Disadvantages:
Can take up to 24h to dry
Limited sample mass (a few kg)
Low surface area of solid
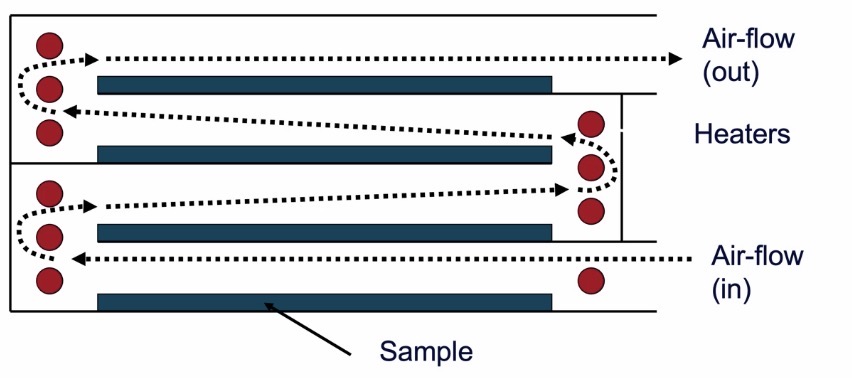
Fluidised-bed Drying
Method
Advantages
Disadvantages
Hot, turbulent air passed in from below the powder bed
If air flow is high enough, particles aerosolise and become fluidised
Filter bag prevents powder particles from leaving the top of the dryer, and has small holes to allow the air out
Advantages:
Very rapid drying (ca. 30 mins)
Energy efficient
Can handle large masses (ca 100s kg)
Can smooth particles
Disadvantages:
Risk of dust explosion
Spray Drying
Method
Advantages
For drying solutions / suspensions
Hot air and solution/suspension passed through a nozzle at the same time
Sample becomes an aerosol
Aerosol droplets dried in a vortex of hot air
As the droplets dry = become denser = falls to the bottom of the spray dryer
Advantages:
Very rapid drying (1-2s for each droplet to dry)
Energy efficient
Can run continuously
Produces spherical particles
Often makes material amorphous = dissolve fast
Freeze Drying
Method
Advantages
Disadvantages
Based on the fact that water has a triple point - where solid, liquid and gas intersect
Water is able to go straight from a solid to a gas = sublimation
Solution/suspension inserted into freeze dryer at atmospheric pressure (105 Pa) and room temperature » point 1 on the diagram
Under these conditions, water is a liquid
The sample is frozen » reduce temperature, keep pressure the same » point 1 to point 2
A vacuum is initiated, causing pressure to drop below 610Pa » point 2 to point 3
Increase temperature back to room temperature » point 3 to point 4
Frozen water sublimes and becomes a gas, which is removed
Advantages:
No rise in temperature, so good for heat sensitive samples
Makes highly porous, amorphous material = very fast dissolution
Disadvantages:
Expensive to operate
Small samples (usually in glass vials)
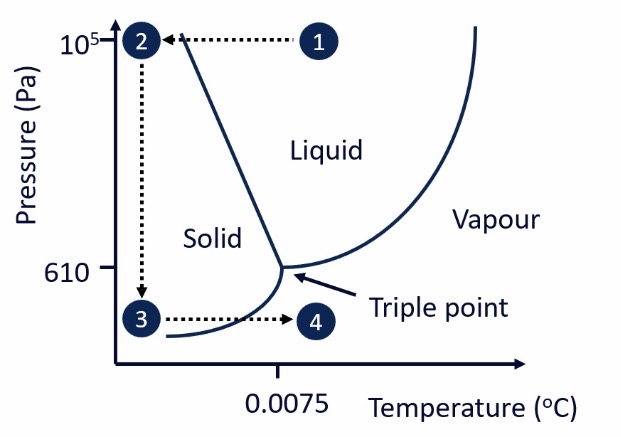
Which is the only drying method where temperature is not increased above room temperature?
Freeze drying
Which solvent, other than water, has a triple point and can be freeze dried
T-butanol
Diluents
What are diluents?
Bulking agents
Added to increase bulk and make the tablet a practical size for compression
Diluents used in direct compression need to have similar particle size to the drug, and good compressibility
With insoluble drugs, need to add hydrophilic diluents
The diluents must have good compatibility with the drug
Diluents
Examples
Lactose monohydrate
Microcrystalline cellulose
Dicalcium phosphate dihydrate
Pregelatinised starch
Mannitol
Calcium sultate
Corn starch
Disintegrants
What are disintegrants?
Facilitate the break up of tablets into individual granules/particles upon contact with water
Mechanisms of action
Wicking - capillary forces promoting rapid water uptake
Swelling
Release of gases upon contact with water
Melting at body temperature
Enzymatic destruction of binder
Disintegrants
Examples
Normal disintegrants
Starches (corn, pre-gelatinised)
Alginates
Super disintegrants
Cross-linked carboxymethylcellulose 2-6% (Ac-di-sol)
Sodium starch glycolate 2-8% (Explotab)
Crospovidone 2 - 6% (Kollidon CL or Polyplasdone)
Disintegrants
When do you use a normal or super disintegrant?
Low solubility = use super disintegrant to speed up disintegration and drug dissolution
Strong granule = use superdisintegrant to speed up disintegration
Disintegrants
When do we add a disintegrant?
Option 1: Add all disintegrant prior to granulation
Option 2 (Preferred/Most common): Split addition
2/3 added prior to granulation
1/3 added to the dry granules before compression
Examples of common excipients used in tablet formulation
Microcrystalline Cellulose (MCC) - 90 microns:
• Excellent compactability and flow
• Limited disintegration properties
Starch 1500
• Adds bulk to the tablets
• Good compactability, flow and lubricity
• Disintegration property
Lubricants
Purpose of lubricants
Examples
Reduces friction between powder and tableting tools
E.g.
Mg stearate / Ca stearate
Polyethylene glycol
Lubricants
When is the lubricants added
Whether tablets are made by:
Direct compression, or
Wet/dry granulation
The lubrication step is essentially the same:
Lubricant is added at the final blending stage, just before compression
Lubricants
Advantages
Reduce friction between powder/granules and dies and punches
Prevent material from sticking to equipment
Ensure smooth tablet ejection
Low shear mixing → avoids excessive coating
Minimum effective amount → typically 0.25–1%
Short mixing time → limits negative effects
Lubricants
What happens if there is too much lubrication?
Particles become over-coated
Results in:
Soft tablets (reduced hardness)
Poor dissolution (water cannot penetrate easily)
This happens because lubricants repel water and weaken particle bonding
Lubricants
What happens if there is too little lubrication?
High friction during compression
Leads to:
Sticking – material sticks to punch faces
Picking – part of the tablet surface is pulled off
Causes defective tablets and equipment issues.
Glidants
Purpose of glidants
Examples
To improve powder flowability
E.g. Colloidal silicon dioxide
Examples of interactions between APIs and excipients
E.g.
Lactose and primary or secondary amines → Maillard reaction
CaHPO, is alkaline, so need to consider pH stability of drug
What must happen to all excipients before direct compression
Must be spray dried to ensure they are spherical, so they mix and flow well
Possible problems after tablet manufacturing
Weight variation
Caused by variation of flow of powder into the die
Leads to variation in drug content
Solution:
Improve flow properties
Reduce compression speed
Possible problems after tablet manufacturing
Segmentation of granules/powder
Leads to variation in drug content
Solution: improve powder flow properties
Possible problems after tablet manufacturing
Capping / Lamination
Caused by excessive elastic recovery
Moisture content may be too high or low
Solution:Add excipients to give more brittle fracture
Possible problems after tablet manufacturing
Sticking
Bits of formulation stick to the punches
Causes by small/irregular particles or high moisture content
Solution: increase the amount of lubricant/lubrication time
Possible problems after tablet manufacturing
Picking
Tablets pick up bits of powder on the surface
Causes by small/irregular particles or high moisture content
Solution: increase the amount of lubricant/lubrication time
Possible problems after tablet manufacturing
Chipping / Cracking
Caused by:
large particle size
high elastic recovery
high humidity
Possible problems after tablet manufacturing
Poor dissolution properties
Caused by:
Particle size
High moisture content
Low binding
Segregation
How are tablets made to be easy to swallow?
To improve swallowability, tablets are often given a special film coating. This coating is formulated to:
Be smooth and slippery
Show low bioadhesion (does not stick to the mouth or throat)
Coating composition
Polymers:
Hydroxypropyl methylcellulose (HPMC)
Polyvinyl alcohol (PVA)
These form a smooth, flexible film with low adhesion
Plasticisers:
Polyethylene glycol (PEG)
Plasticisers increase flexibility and prevent cracking
Colorants:
Improve appearance and aid product identification
2 types of capsules
Hard (shell) capsule
Two piece capsules which include a "body" and "cap"
Capsule materials: Gelatin or Hydroxypropyl methylcellulose (HPMC) or Starch
Contents may be solids or liquids
Three stage manufacturing process: i) shell, ii) contents, ili) filling
Soft (shell) capsule
One piece
Capsule material: Gelatin
Contents are liquids
Two stage manufacturing process: i) contents, ii) shell manufacture and filling combined
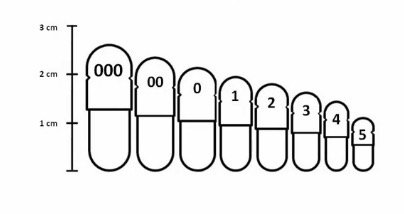
Capsule Sizes
There are 8 standard capsule sizes, ranging from: 000 (largest) → 5 (smallest)
As the capsule size number increases, the capsule volume decreases
How to estimate fill weight for hard capsules
Fill weight ≈ capsule body volume × tapped bulk density
Tapped bulk density is used because it reflects how powder behaves after settling, which is closer to real capsule filling conditions.
How to estimate fill volume for soft capsules
Fill volume depends on the density of the liquid in the capsule
Hard Capsules
How are hard gelatin capsule shells manufactured?
Gelatin dissolved in hot water (60°C to 70°C)
Colour is added as necessary
Stainless steel pins (fingers) dipped into the gelatin solution
the coating on the pins will become one part of the capsule
the pins are rotated to ensure constant thickness of coating
the coating is dried, removed and trimmed
the two halves of the capsules are joined together
Hard Capsules
What is the standard moisture content for hard gelatin capsules?
What is the recommended storage conditions?
Standard moisture content: 13% to 16% w/w
Storage conditions: 50% relative humidity at 21°C
Hard Capsules
Why do we use hard capsules compared to tablets?
No water involved in the dry powder mix → prevent hydrolysis
No heat involved in the dry powder mix → good for heat-sensitive drugs
No compression involved in filling of dry powder mix → avoid possible changes of physical state (amorphous v crystalline, polymorphic changes) → prevent change in dissolution profile
Hard Capsules
Why may HPMC be used as a capsule material instead of gelatin?
Used to improve performance and stability of formulations, especially for:
Poorly soluble APIs » better solubilisation
Aldehyde-containing formulations » avoids gelatin cross-linking and disintegration issues
Modified-release formulations
Moisture-sensitive drugs » lower moisture content than gelatin, better stability)
Soft Capsules
How are soft gelatin capsule shells manufactured?
Preparation of the ribbon
Gelatin dissolved in hot water
Plasticisers added (eg glycerol, sorbitol, PEG, propylene glycol)
Colour is added as necessary
A gel "ribbon" is formed
Preparation and filling of the capsules
Two gel ribbons are placed on the rollers
Ribbons are partially sealed to provide a "cup"
The liquid is added to the cup
The ribbons are sealed above the fill, making an intact capsule
The capsules are dried
Soft Capsules
Why do we use soft shell capsules compared to tablets?
For poorly water soluble drugs
Drug is in solution in the fill
Avoids dissolution step in the gastro-intestinal tract
Promotes bioavailability, especially if in a self-emulsifying formulation
Excipients in a capsule filling
Diluents (filler)
Lubricants, which reduce powder-to-metal adhesion
Glidants, which improve powder flow
Wetting agents, which improve water penetration
Disintegrants, which produce disruption of the powder mass
Hard Capsules
Limitations of filling for hard capsules
React with shell material
Contains high levels of moisture
Volume of unit dose exceeds the capsule size available
Hard Capsules
Different types of fillings for hard capsules
Powders
Granules » improve mixing, reduce segregation
Pellets » enable controlled release and combination drugs
Mini-tablets » different drugs or release rates in one capsule
Non-aqueous liquids » oils or oil/surfactant systems for very poorly water-soluble drugs; may be liquid or molten-filled solids