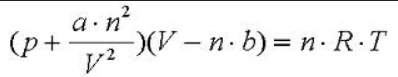Ch. 8 - Gases
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
Barometer
used to measure atmosphereic pressure
Patm = 760 mmHg
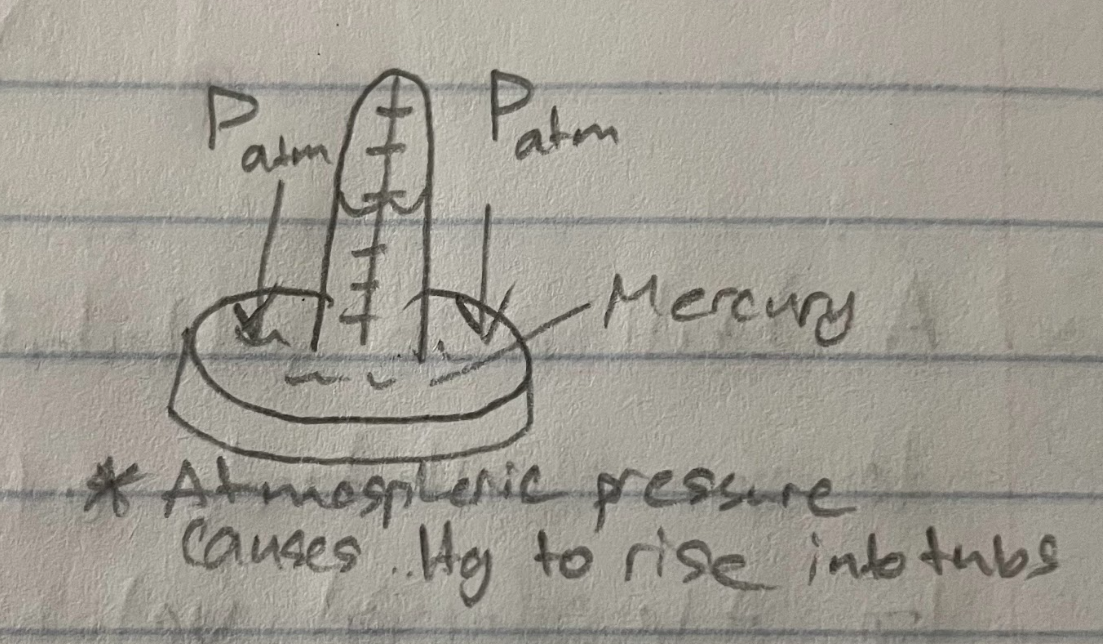
Manometer
used to measure pressure of a gas sample
closed end (manometer)
pressure relative to a vacum
Δheight = Pgas
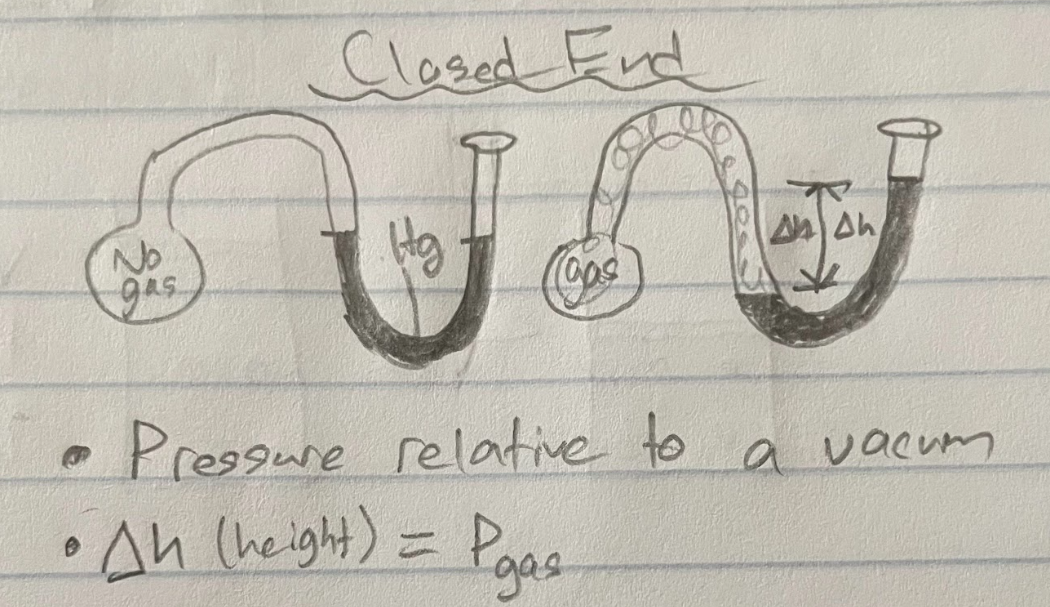
open end (manometer)
Pressure relative to Patm
Pgas = Patm ± Δheight
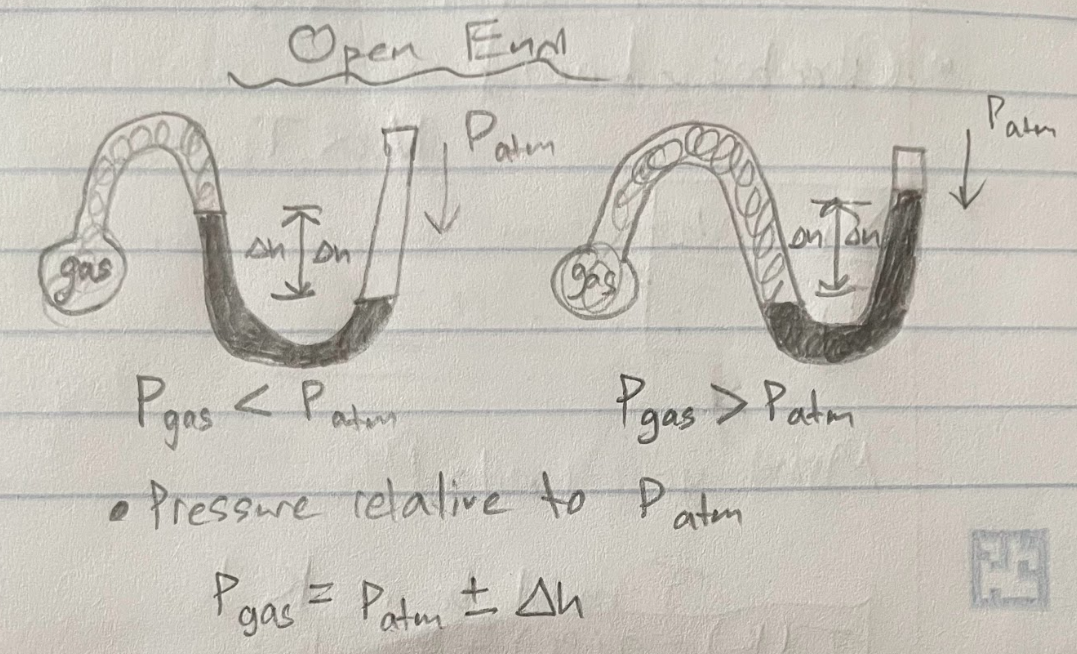
Pascal (Units of Pressure)
1 Pa = 1 N/m²
Atmosphere (Units of Pressure)
average atomospheric pressure
1 atm = 101.3 kPa = 1.013×105 Pa = 1.013 bar
Boyle’s Law
P1V1 = P2V2
Charles’s Law
V1 / T1 = V2 / T2 (T in Kelvin)
Avogadro’s Law
V1 / n1 = V2 / n2
Ideal Gas Law
PV=nRT
R = 0.0821 L*atm/K*mol “universal gas constant”
P = usually in atm
STP
1 atm, 273 K
Density = m/V
n = m/MM
D = m/v = P*MM / R*T
Dalton’s Law in terms of pressure
Ptot = PA + PB + PC…..
Dalton’s Law in terms of moles
Ptot = ntotRT / V
Dalton’s Law in terms of mole fraction
PA = XAPtot PA = (nA/ntot) * Ptot
Application of Dalton’s Law: Gas stoichiometry
Rxn that produces gas
Gas transported to the collector
Gas displaces water in the collector
Vapor pressure of water: water molecules escape surface and create a small partial pressure (Temp dependent)
The Postulates (KMT)
1.) Particle volume is assumed to be 0: gas particles are treated as “point masses”
2.) Gas particles are in constant, random motion collisions w/ the walls (causes pressure)
3.) The particles exert no forces on each other (not always true)
4.) The avg KE of the particles in proportional to Temp (K)
Pressure and Volume (KMT)
V decreases - P increases (Collisions increase (more frequent; NOT faster))
Pressure and Temp (KMT)
T increases - P increases (particles move faster and collisions are more frequent w/ more force)
Volume and Temp (KMT)
T increases - V increases (collisions more frequent and forceful)
Volume and Moles (KMT)
n increases - V increases (more gas particles; more collisions)
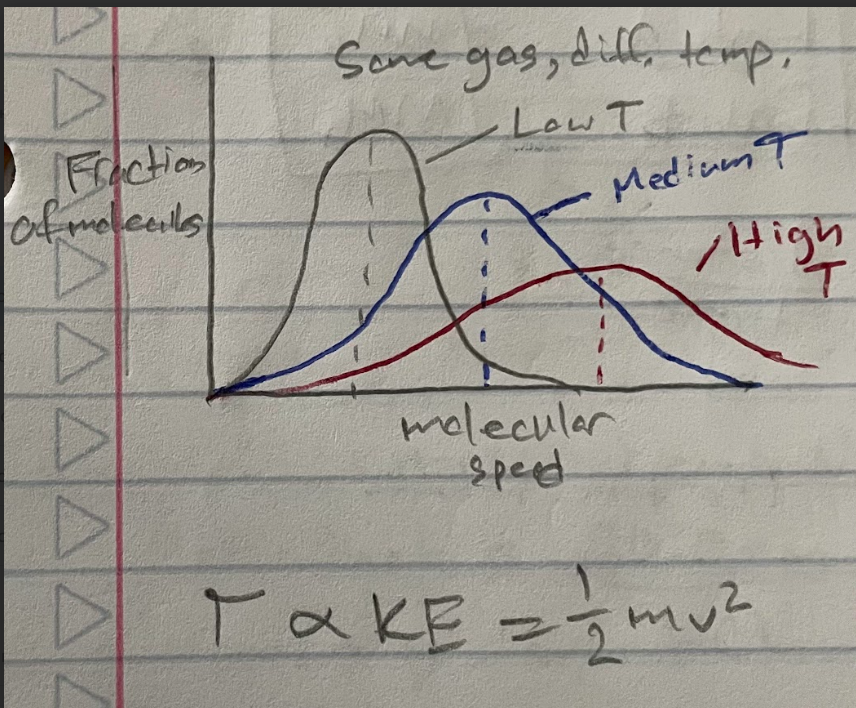
Same gas, different T
T and KE are proportional
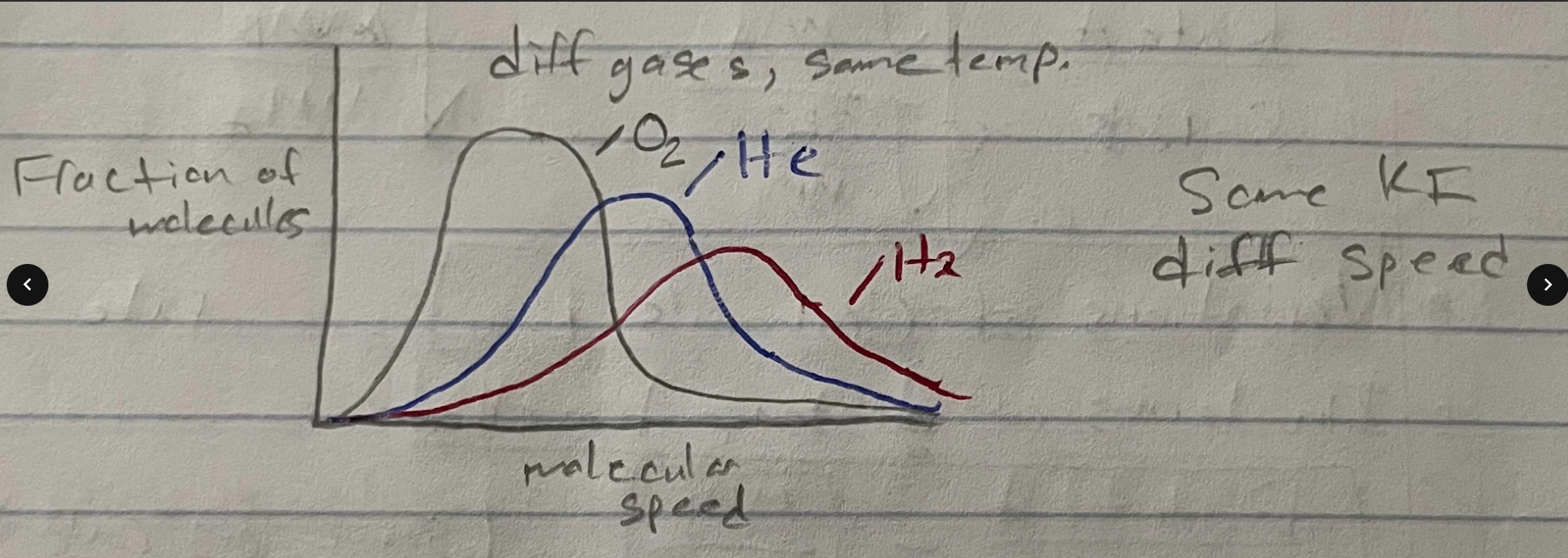
different gases, same T
At a given T, the KEavg will be constant, lighter gases will have higher avg speed
The graph shows gases with same KE, different speeds
Equation relating molecular speed and temp (rms speed)
R = 8.314 J/K*mol
MM = in kg/mol
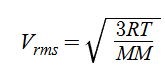
Grahams Law of Effusion/Diffusion
Effusion: escape of a gas from a small hole
Diffusion: movement of a gas through another gas
Equations relates rates
MM in g/mol
rate in mL
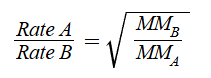
In terms of distance travelled
same as rate
no time mentioned
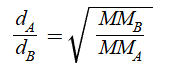
In terms of time
MMA and MMB switch
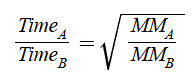
Real gases
deviations from ideal gas behavior
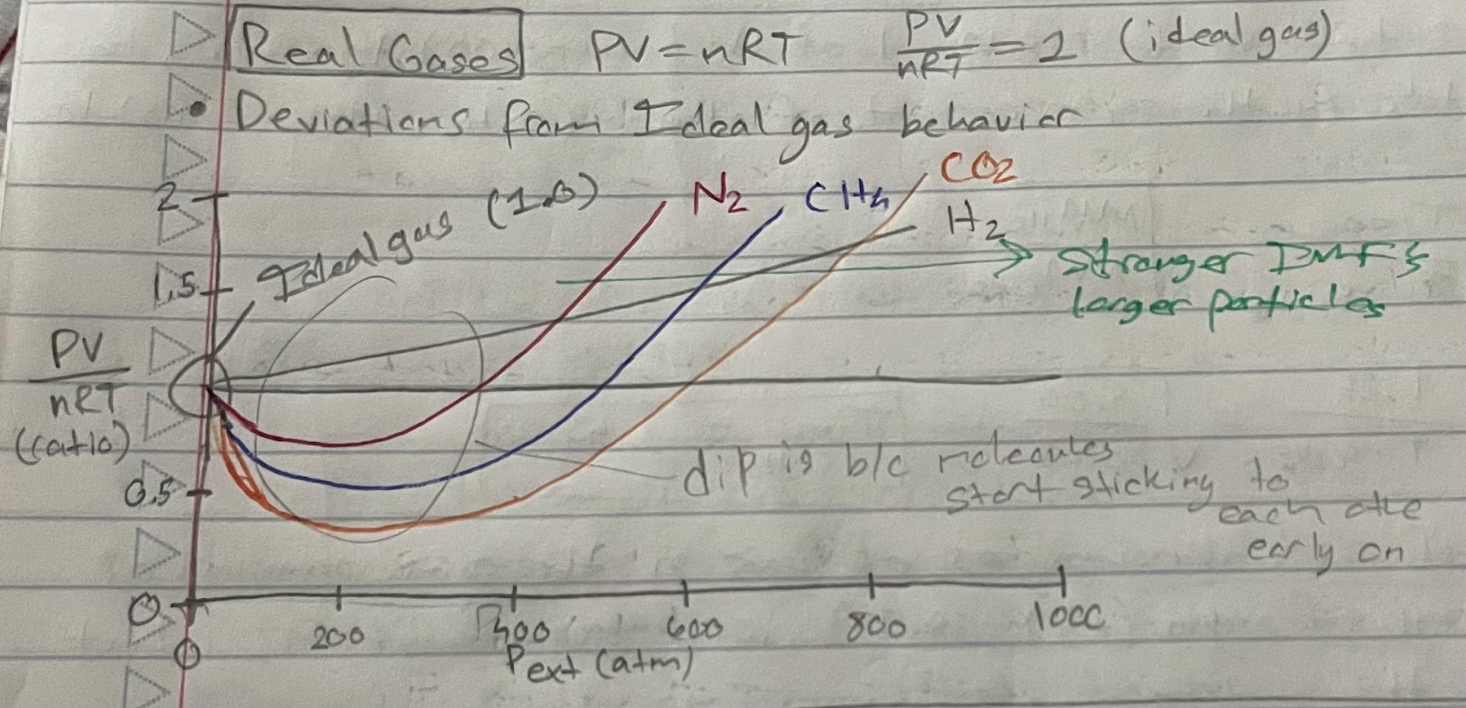
When do real gases behave most like ideal gases?
Low P: Gas particles very far apart from each other
minimizes interactions
Vparticles < < Vcontainer
High T: Gas particles moving very fast
Spend little time interacting with each other
Van der Waals equation (for REAL gases)
do not need to ever use, but understand the components
a: adjusts for interparticle attractive forces (IMF increases; a increases)
b: Adjusts V to account for particle volume (repulsive forces (volume); the size of particles increases, b increases)