The equilibrium constant Kp and controlling the position of equilibrium
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
What is the mole fraction?
The number of moles of the specific gas / total number of moles in gas mixture
What is partial pressure?
The mole fraction of a specific gas x the total pressure
What does the sum of all the partial pressure equal?
The total pressure
What will the total of the mole fractions always be?
1
What is the equation for Kp?
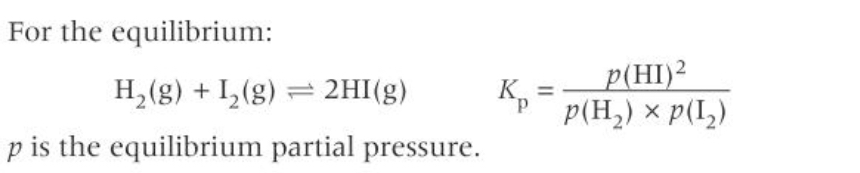
What does Kp only include?
Gases - only gases have partial pressures
Other species must be ignored
What happens to K at a set temperature?
K is constant and doesn’t change despite any modifications to concentration, pressure of presence of a catalyst
What does cause K to change?
If the temperature is changed
If the forward reaction is exothermic, what happens if the temperature is increases? in terms of Kp
Equilibrium constant decreases
Raising the temperature decreases the equilibrium yield of products - equilibrium shifts to the left
numerator of Kp expression increases more
denominator of Kp increases to restore Kp
If the forward reaction is endothermic, what happens if the temperature is increased?
Equilibrium constant increases
Increases equilibrium yield of products
Does Kp change with pressure?
NO
only temperature